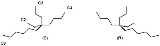
Cryptochirality
Encyclopedia
Cryptochirality in stereochemistry
is a special case of chirality
where due to the electronic properties of the chiral molecule its specific rotation
is non-measurable. The term was introduced by K. Mislow in 1977.
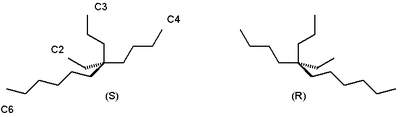
For example the alkane
5-ethyl-5-propylundecane or (n-butyl)ethyl(n-hexyl)(n-propyl)methane found in certain species of Phaseolus vulgaris has two enantiomeric forms without any observable optical rotation
 It is still possible to distinguish between the two enantiomers by asymmetric synthesis with the (S)-alkane 3 in an autocatalytic system of the achiral aldehyde
It is still possible to distinguish between the two enantiomers by asymmetric synthesis with the (S)-alkane 3 in an autocatalytic system of the achiral aldehyde
1 and achiral Diisopropylzinc
2 to the chiral (R)-alcohol
4 with enantiomeric excess
94%:
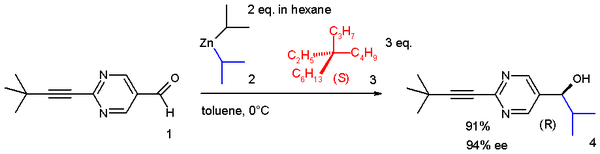 For chiral induction to be possible C-H bonds in the alkane are believed to interact with the pi electrons of the aldehyde.
For chiral induction to be possible C-H bonds in the alkane are believed to interact with the pi electrons of the aldehyde.
Cryptochirality also occurs in polymer
ic systems growing from chiral initiator
s for example in dendrimer
s with lobes of different size attached to a central core.
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
is a special case of chirality
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
where due to the electronic properties of the chiral molecule its specific rotation
Specific rotation
In stereochemistry, the specific rotation of a chemical compound [α] is defined as the observed angle of optical rotation α when plane-polarized light is passed through a sample with a path length of 1 decimeter and a sample concentration of 1 gram per 1 millilitre. It is the main property used to...
is non-measurable. The term was introduced by K. Mislow in 1977.
For example the alkane
Alkane
Alkanes are chemical compounds that consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds without any cycles...
5-ethyl-5-propylundecane or (n-butyl)ethyl(n-hexyl)(n-propyl)methane found in certain species of Phaseolus vulgaris has two enantiomeric forms without any observable optical rotation

Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
1 and achiral Diisopropylzinc
Diisopropylzinc
Diisopropylzinc is an organozinc compound with the chemical formulaZnC6H14....
2 to the chiral (R)-alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
4 with enantiomeric excess
Enantiomeric excess
The enantiomeric excess of a substance is a measure of how pure it is. In this case, the impurity is the undesired enantiomer .-Definition:...
94%:

Cryptochirality also occurs in polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
ic systems growing from chiral initiator
Initiator
An initiator can refer to:* A person that takes an initiative in making something happen.* Modulated neutron initiator, a neutron source used in some nuclear weapons...
s for example in dendrimer
Dendrimer
Dendrimers are repetitively branched molecules. The name comes from the Greek word "δένδρον" , which translates to "tree". Synonymous terms for dendrimer include arborols and cascade molecules. However, dendrimer is currently the internationally accepted term. A dendrimer is typically symmetric...
s with lobes of different size attached to a central core.

