
Critical point (thermodynamics)
Encyclopedia

Physical chemistry
Physical chemistry is the study of macroscopic, atomic, subatomic, and particulate phenomena in chemical systems in terms of physical laws and concepts...
, thermodynamics
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
, chemistry
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
and condensed matter physics
Condensed matter physics
Condensed matter physics deals with the physical properties of condensed phases of matter. These properties appear when a number of atoms at the supramolecular and macromolecular scale interact strongly and adhere to each other or are otherwise highly concentrated in a system. The most familiar...
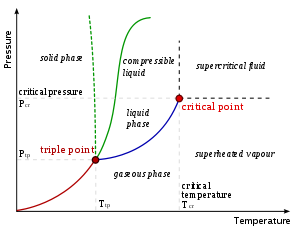
, a critical point, also called a critical state, specifies the conditions (temperature, pressure and sometimes composition) at which a phase
Phase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
boundary ceases to exist. There are multiple types of critical points such as vapor–liquid critical points and liquid–liquid critical points.
Pure substances: vapor–liquid critical point
The term "critical point" is sometimes used to denote specifically the vapor–liquid critical point of a material. The vapor–liquid critical point denotes the conditions above which distinct liquidLiquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
and gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
phases do not exist.
Pascal (unit)
The pascal is the SI derived unit of pressure, internal pressure, stress, Young's modulus and tensile strength, named after the French mathematician, physicist, inventor, writer, and philosopher Blaise Pascal. It is a measure of force per unit area, defined as one newton per square metre...
(3200 PSIA or 218 atm
Atmosphere (unit)
The standard atmosphere is an international reference pressure defined as 101325 Pa and formerly used as unit of pressure. For practical purposes it has been replaced by the bar which is 105 Pa...
).
As the critical temperature is approached, the properties of the gas and liquid phases approach one another, resulting in only one phase at the critical point: a homogeneous supercritical fluid
Supercritical fluid
A supercritical fluid is any substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist. It can effuse through solids like a gas, and dissolve materials like a liquid...
. The heat of vaporization is zero at and beyond this critical point, so there is no distinction between the two phases. Above the critical temperature a liquid cannot be formed by an increase in pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
, but with enough pressure a solid may be formed. The critical pressure is the vapor pressure
Vapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
at the critical temperature. On the diagram showing the thermodynamic properties for a given substance, the point at which critical temperature and critical pressure meet is called the critical point of the substance. The critical molar volume
Molar volume
The molar volume, symbol Vm, is the volume occupied by one mole of a substance at a given temperature and pressure. It is equal to the molar mass divided by the mass density...
is the volume of one mole of material at the critical temperature and pressure.
Critical properties vary from material to material, just as is the case for the melting point
Melting point
The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure...
and boiling point
Boiling point
The boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid....
. Critical properties for many pure substances are readily available in the literature. Obtaining critical properties for mixtures is somewhat more problematic.
Mathematical definition
For pure substances, there is an inflection pointInflection point
In differential calculus, an inflection point, point of inflection, or inflection is a point on a curve at which the curvature or concavity changes sign. The curve changes from being concave upwards to concave downwards , or vice versa...
in the critical isotherm (or temperature contour line
Contour line
A contour line of a function of two variables is a curve along which the function has a constant value. In cartography, a contour line joins points of equal elevation above a given level, such as mean sea level...
) on a pV diagram. This means that at the critical point:
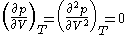
This relation can be used to evaluate two parameters for an equation of state in terms of the critical properties.
Sometimes a set of reduced properties
Reduced properties
In thermodynamics, the reduced properties of a fluid are a set of state variables normalized by the fluid's state properties at its critical point...
are defined in terms of the critical properties, i.e.:

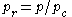

where
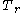 is the reduced temperature,
is the reduced temperature, 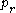 is the reduced pressure,
is the reduced pressure,  is the reduced volume, and
is the reduced volume, and  is the universal gas constant.
is the universal gas constant.Principle of corresponding states
Critical variables are useful for rewriting a varied equation of state into one that applies to all materials. The effect is similar to a normalizing constantNormalizing constant
The concept of a normalizing constant arises in probability theory and a variety of other areas of mathematics.-Definition and examples:In probability theory, a normalizing constant is a constant by which an everywhere non-negative function must be multiplied so the area under its graph is 1, e.g.,...
. The principle of corresponding states
Theorem of corresponding states
According to van der Waals, the theorem of corresponding states indicates that all fluids, when compared at the same reduced temperature and reduced pressure, have approximately the same compressibility factor and all deviate from ideal gas behavior to about the same degree.Material constants that...
indicates that substances at equal reduced pressures and temperatures have equal reduced volumes. This relationship is approximately true for many substances, but becomes increasingly inaccurate for large values of pr
Table of liquid–vapor critical temperature and pressure for selected substances
| Substance | Critical temperature | Critical pressure |
|---|---|---|
| Argon Argon Argon is a chemical element represented by the symbol Ar. Argon has atomic number 18 and is the third element in group 18 of the periodic table . Argon is the third most common gas in the Earth's atmosphere, at 0.93%, making it more common than carbon dioxide... |
||
| Ammonia Ammonia Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or... |
||
| Bromine Bromine Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826... |
||
| Caesium Caesium Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature... |
||
| Chlorine Chlorine Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine... |
||
| Ethanol Ethanol Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a... |
241 °C (514 K) | 62.18 atm (63 bar, 6,300 kPa) |
| Fluorine Fluorine Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic... |
||
| Helium Helium Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table... |
||
| Hydrogen Hydrogen Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly... |
||
| Krypton Krypton Krypton is a chemical element with the symbol Kr and atomic number 36. It is a member of Group 18 and Period 4 elements. A colorless, odorless, tasteless noble gas, krypton occurs in trace amounts in the atmosphere, is isolated by fractionally distilling liquified air, and is often used with other... |
||
| CH4 Methane Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel... |
||
| Neon Neon Neon is the chemical element that has the symbol Ne and an atomic number of 10. Although a very common element in the universe, it is rare on Earth. A colorless, inert noble gas under standard conditions, neon gives a distinct reddish-orange glow when used in either low-voltage neon glow lamps or... |
||
| Nitrogen Nitrogen Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere... |
||
| Oxygen Oxygen Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition... |
||
| CO2 Carbon dioxide Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom... |
||
| N2O Nitrous oxide Nitrous oxide, commonly known as laughing gas or sweet air, is a chemical compound with the formula . It is an oxide of nitrogen. At room temperature, it is a colorless non-flammable gas, with a slightly sweet odor and taste. It is used in surgery and dentistry for its anesthetic and analgesic... |
||
| H2SO4 Sulfuric acid Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates... |
||
| Xenon Xenon Xenon is a chemical element with the symbol Xe and atomic number 54. The element name is pronounced or . A colorless, heavy, odorless noble gas, xenon occurs in the Earth's atmosphere in trace amounts... |
||
| Lithium Lithium Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly... |
||
| Mercury Mercury (element) Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum... |
||
| Sulfur Sulfur Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow... |
||
| Iron Iron Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust... |
||
| Gold Gold Gold is a chemical element with the symbol Au and an atomic number of 79. Gold is a dense, soft, shiny, malleable and ductile metal. Pure gold has a bright yellow color and luster traditionally considered attractive, which it maintains without oxidizing in air or water. Chemically, gold is a... |
||
| Aluminium Aluminium Aluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances.... |
||
| Water Water Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a... |
||
History
The existence of a critical point was first discovered by Thomas AndrewsThomas Andrews (scientist)
Thomas Andrews FRS was an Irish chemist and physicist who did important work on phase transitions between gases and liquids.-Life:Andrews was born in Belfast, Ireland where his father was a linen merchant...
in 1869 for carbon dioxide. He showed that CO2 could be liquefied at 31oC at a pressure of 73 atm, but not at slightly higher temperatures even under very much higher pressures (to 3000 atm).
Mixtures: liquid–liquid critical point
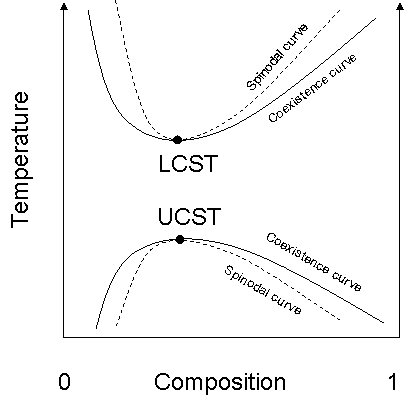
Upper critical solution temperature
The upper critical solution temperature or upper consolute temperature is the critical temperature above which the components of a mixture are miscible in all proportions. The word upper indicates that the UCST is an upper bound to a temperature range of partial miscibility, or miscibility for...
, or UCST, which denotes the warmest point at which cooling will induce phase separation, and the lower critical solution temperature
Lower critical solution temperature
The lower critical solution temperature or lower consolute temperature is the critical temperature below which the components of a mixture are miscible for all compositions...
, which denotes the coolest point at which heating will induce phase separation.
Mathematical definition
From a theoretical standpoint, the liquid–liquid critical point represents the temperature-concentration extremum of the spinodal curve (this can be seen in the figure to the right). Thus in a two-component system it must satisfy two conditions. First is the condition of the spinodal curve, which is that the second derivative of the free energy with respect to concentration must equal zero. The second condition is the extremum condition that the third derivative of the free energy with respect to concentration must also equal zero, or that the derivative of the spinodal temperature with respect to concentration must equal zero.In renormalization group theory
According to renormalization groupRenormalization group
In theoretical physics, the renormalization group refers to a mathematical apparatus that allows systematic investigation of the changes of a physical system as viewed at different distance scales...
theory, the defining property of criticality is that the natural length scale
Length scale
In physics, length scale is a particular length or distance determined with the precision of one order of magnitude. The concept of length scale is particularly important because physical phenomena of different length scales cannot affect each other and are said to decouple...
characteristic of the structure of the physical system, the so-called correlation length ξ, becomes infinite. This effect is the cause of the critical opalescence
Critical opalescence
Critical opalescence is a phenomenon which arises in the region of a continuous, or second-order, phase transition. Originally reported by Thomas Andrews in 1869 for the liquid-gas transition in carbon dioxide, many other examples have been discovered since. The phenomenon is most commonly...
that is seen as the liquid–liquid critical point is approached in a binary fluid mixture. There are also lines in phase space
Phase space
In mathematics and physics, a phase space, introduced by Willard Gibbs in 1901, is a space in which all possible states of a system are represented, with each possible state of the system corresponding to one unique point in the phase space...
along which this happens: these are critical lines.
In equilibrium systems the critical point is reached only by tuning a control parameter precisely. However, in some non-equilibrium
Non-equilibrium thermodynamics
Non-equilibrium thermodynamics is a branch of thermodynamics that deals with systems that are not in thermodynamic equilibrium. Most systems found in nature are not in thermodynamic equilibrium; for they are changing or can be triggered to change over time, and are continuously and discontinuously...
systems the critical point is an attractor
Attractor
An attractor is a set towards which a dynamical system evolves over time. That is, points that get close enough to the attractor remain close even if slightly disturbed...
of the dynamics in a manner that is robust with respect to system parameters, a phenomenon referred to as self-organized criticality
Self-organized criticality
In physics, self-organized criticality is a property of dynamical systems which have a critical point as an attractor. Their macroscopic behaviour thus displays the spatial and/or temporal scale-invariance characteristic of the critical point of a phase transition, but without the need to tune...
.
The critical point is described by a conformal field theory
Conformal field theory
A conformal field theory is a quantum field theory that is invariant under conformal transformations...
.
See also
- Conformal field theoryConformal field theoryA conformal field theory is a quantum field theory that is invariant under conformal transformations...
- Critical exponents
- Critical phenomenaCritical phenomenaIn physics, critical phenomena is the collective name associated with thephysics of critical points. Most of them stem from the divergence of thecorrelation length, but also the dynamics slows down...
- Joback methodJoback methodThe Joback method predicts eleven important and commonly used pure component thermodynamic properties from molecular structure only.- Group Contribution Method :The Joback method is a group contribution method...
, Klincewicz methodKlincewicz methodIn thermodynamic theory, the Klincewicz method is a predictive method based both on group contributions and on a correlation with some basic molecular properties...
, Lydersen methodLydersen methodThe Lydersen method is a group contribution method for the estimation of critical properties temperature , pressure and volume . The Lydersen method is the prototype for and ancestor of many new models like Joback, Klincewicz,Ambrose,...
(Estimation of critical temperature, pressure, and volume from molecular structure) - Lower critical solution temperatureLower critical solution temperatureThe lower critical solution temperature or lower consolute temperature is the critical temperature below which the components of a mixture are miscible for all compositions...
- Percolation thresholds
- Phase transitionPhase transitionA phase transition is the transformation of a thermodynamic system from one phase or state of matter to another.A phase of a thermodynamic system and the states of matter have uniform physical properties....
- Rushbrooke inequalityRushbrooke inequalityIn statistical mechanics, the Rushbrooke inequality relates the critical exponents of a magnetic system which exhibits a first-order phase transition in the thermodynamic limit for non-zero temperature T....
- Scale invarianceScale invarianceIn physics and mathematics, scale invariance is a feature of objects or laws that do not change if scales of length, energy, or other variables, are multiplied by a common factor...
- Self-organized criticalitySelf-organized criticalityIn physics, self-organized criticality is a property of dynamical systems which have a critical point as an attractor. Their macroscopic behaviour thus displays the spatial and/or temporal scale-invariance characteristic of the critical point of a phase transition, but without the need to tune...
- Supercritical fluidSupercritical fluidA supercritical fluid is any substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist. It can effuse through solids like a gas, and dissolve materials like a liquid...
, Supercritical dryingSupercritical dryingSupercritical drying is a process to remove liquid in a precisely controlled way. It is useful in the production of microelectromechanical systems , the drying of spices, the production of aerogel, and in the preparation of biological specimens for scanning electron microscopy.As the substance in...
, Supercritical water oxidationSupercritical water oxidationSupercritical water oxidation or SCWO is a process that occurs in water at temperatures and pressures above a mixture's thermodynamic critical point. Under these conditions water becomes a fluid with unique properties that can be used to advantage in the destruction of hazardous wastes such as PCBs... - Tricritical pointTricritical pointIn condensed matter physics, dealing with the macroscopic physical properties of matter, a tricritical point is a point in the phase diagram of a system at whichthree-phase coexistence terminates...
- Triple pointTriple pointIn thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases of that substance coexist in thermodynamic equilibrium...
- Upper critical solution temperatureUpper critical solution temperatureThe upper critical solution temperature or upper consolute temperature is the critical temperature above which the components of a mixture are miscible in all proportions. The word upper indicates that the UCST is an upper bound to a temperature range of partial miscibility, or miscibility for...
- Widom scalingWidom scalingWidom scaling is a hypothesis in statistical mechanics regarding the free energy of a magnetic system near its critical point which leads to the critical exponents becoming no longer independent so that they can be parameterized in terms of two values...
External links
- Hagen KleinertHagen KleinertHagen Kleinert is Professor of Theoretical Physics at the Free University of Berlin, Germany , at theWest University of Timişoara, at thein Bishkek. He is also of the...
and Verena Schulte-Frohlinde, Critical Properties of φ4-Theories, World Scientific (Singapur, 2001); Paperback ISBN 981-02-4658-7 (readable online here)

