
Coordination geometry
Encyclopedia
The term coordination geometry is used in a number of related fields of chemistry and solid state chemistry/physics.
s that are bonded to the central atom in a molecule
or a coordination complex. The geometrical arrangement will vary according to the number and type of ligands bonded to the metal centre, and to the coordination preference of the central atom, typically a metal in a coordination complex. The number of atoms bonded, (i.e. the number of σ-bonds between central atom and ligands) is termed the coordination number
.
The geometrical pattern can be described as a polyhedron
where the vertices of the polyhedron are the centres of the coordinating atoms in the ligands.
The coordination preference of a metal often varies with its oxidation state. The number of coordination bonds (coordination number
) can vary from two as high as 20 in Th(η5-C5H5)4.
One of the most common coordination geometries is octahedral
, where six ligands are coordinated to the metal in a symmetrical distribution, leading to the formation of an octahedron
if lines were drawn between the ligands. Other common coordination geometries are tetrahedral
and square planar.
Crystal field theory
may be used to explain the relative stabilities of transition metal compounds of different coordination geometry, as well as the presence or absence of paramagnetism
.
VSEPR may be used for complexes of main group element
to predict geometry.
with the body centred cubic (bcc) structure each atom has eight nearest neighbours in a cubic geometry. In metals
with the face centred cubic (fcc) structure each atom has twelve nearest neighbours in a cuboctahedral
geometry.
as part of their IUPAC nomenclature of inorganic chemistry 2005 recommendations
to describe the geometry around an atom in a compound.
IUCr have proposed a symbol which is shown as a superscript in square brackets in the chemical formula. For example CaF2 would be Ca[8cb]F2[4t], where [8cb] means cubic coordination and [4t] means tetrahedral. The equivalent symbols in IUPAC are CU−8 and T−4 respectively.
The IUPAC symbol is applicable to complexes and molecules whereas the IUCr proposal applies to crystalline solids.
Molecules
The coordination geometry of an atom is the geometrical pattern formed by atoms around the central atom.Inorganic coordination complexes
In the field of inorganic coordination complexes it is the geometrical pattern formed by the atoms in the ligandLigand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s that are bonded to the central atom in a molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
or a coordination complex. The geometrical arrangement will vary according to the number and type of ligands bonded to the metal centre, and to the coordination preference of the central atom, typically a metal in a coordination complex. The number of atoms bonded, (i.e. the number of σ-bonds between central atom and ligands) is termed the coordination number
Coordination number
In chemistry and crystallography, the coordination number of a central atom in a molecule or crystal is the number of its nearest neighbours. This number is determined somewhat differently for molecules and for crystals....
.
The geometrical pattern can be described as a polyhedron
Polyhedron
In elementary geometry a polyhedron is a geometric solid in three dimensions with flat faces and straight edges...
where the vertices of the polyhedron are the centres of the coordinating atoms in the ligands.
The coordination preference of a metal often varies with its oxidation state. The number of coordination bonds (coordination number
Coordination number
In chemistry and crystallography, the coordination number of a central atom in a molecule or crystal is the number of its nearest neighbours. This number is determined somewhat differently for molecules and for crystals....
) can vary from two as high as 20 in Th(η5-C5H5)4.
One of the most common coordination geometries is octahedral
Octahedral molecular geometry
In chemistry, octahedral molecular geometry describes the shape of compounds where in six atoms or groups of atoms or ligands are symmetrically arranged around a central atom, defining the vertices of an octahedron...
, where six ligands are coordinated to the metal in a symmetrical distribution, leading to the formation of an octahedron
Octahedron
In geometry, an octahedron is a polyhedron with eight faces. A regular octahedron is a Platonic solid composed of eight equilateral triangles, four of which meet at each vertex....
if lines were drawn between the ligands. Other common coordination geometries are tetrahedral
Tetrahedral molecular geometry
In a tetrahedral molecular geometry a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are cos−1 ≈ 109.5° when all four substituents are the same, as in CH4. This molecular geometry is common throughout the first...
and square planar.
Crystal field theory
Crystal field theory
Crystal field theory is a model that describes the electronic structure of transition metal compounds, all of which can be considered coordination complexes. CFT successfully accounts for some magnetic properties, colours, hydration enthalpies, and spinel structures of transition metal complexes,...
may be used to explain the relative stabilities of transition metal compounds of different coordination geometry, as well as the presence or absence of paramagnetism
Paramagnetism
Paramagnetism is a form of magnetism whereby the paramagnetic material is only attracted when in the presence of an externally applied magnetic field. In contrast with this, diamagnetic materials are repulsive when placed in a magnetic field...
.
VSEPR may be used for complexes of main group element
Main group element
In chemistry and atomic physics, main group elements are elements in groups whose lightest members are represented by helium, lithium,...
to predict geometry.
Crystallography usage
In a crystal structure the coordination geometry of an atom is the geometrical pattern of coordinating atoms where the definition of coordinating atoms depends on the bonding model used. For example in the rock salt ionic structure each sodium atom has six near neighbour chloride ions in an octahedral geometry and each chloride has similarly six near neighbour sodium ions in an octahedral geometry. In metalsPeriodic table (crystal structure)
The structures of metallic elements adopted at standard temperatures and pressures are color coded and shown below, the only exception is mercury, Hg, which is a liquid and the structure refers to the low temperature form. The melting points of the metals is shown above the element symbol...
with the body centred cubic (bcc) structure each atom has eight nearest neighbours in a cubic geometry. In metals
Periodic table (crystal structure)
The structures of metallic elements adopted at standard temperatures and pressures are color coded and shown below, the only exception is mercury, Hg, which is a liquid and the structure refers to the low temperature form. The melting points of the metals is shown above the element symbol...
with the face centred cubic (fcc) structure each atom has twelve nearest neighbours in a cuboctahedral
Cuboctahedron
In geometry, a cuboctahedron is a polyhedron with eight triangular faces and six square faces. A cuboctahedron has 12 identical vertices, with two triangles and two squares meeting at each, and 24 identical edges, each separating a triangle from a square. As such it is a quasiregular polyhedron,...
geometry.
Table of coordination geometries
A table of the coordination geometries encountered is shown below with examples of their occurrence in complexes found as discrete units in compounds and coordination spheres around atoms in crystals (where there is no discrete complex).| Coordination number | Geometry | Examples of discrete (finite) complex | Examples in crystals | |
|---|---|---|---|---|
| 2 | linear |  |
Ag(CN)2− in KAg(CN)2 | Ag in silver cyanide Silver cyanide Silver cyanide is the chemical compound with the formula AgCN. This white solid forms upon treatment of solutions containing Ag+ with cyanide. This precipitation step is used in some schemes to recover silver from solution... , Au in AuI |
| 3 | trigonal planar | 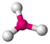 |
Cu(CN)32− in Na2Cu(CN)3.3H2O | O in TiO2 Titanium dioxide Titanium dioxide, also known as titanium oxide or titania, is the naturally occurring oxide of titanium, chemical formula . When used as a pigment, it is called titanium white, Pigment White 6, or CI 77891. Generally it comes in two different forms, rutile and anatase. It has a wide range of... rutile structure |
| 4 | tetrahedral geometry |  |
CoCl42− | Zn and S in zinc sulfide Zinc sulfide Zinc sulfide is a inorganic compound with the formula ZnS. ZnS is the main form of zinc in nature, where it mainly occurs as the mineral sphalerite... , Si in silicon dioxide Silicon dioxide The chemical compound silicon dioxide, also known as silica , is an oxide of silicon with the chemical formula '. It has been known for its hardness since antiquity... |
| 4 | square planar |  |
AgF4− | CuO Copper(II) oxide Copper oxide or cupric oxide is the higher oxide of copper. As a mineral, it is known as tenorite.-Chemistry:It is a black solid with an ionic structure which melts above 1200 °C with some loss of oxygen... |
| 5 | trigonal bipyramidal | 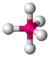 |
SnCl5− | |
| 5 | square pyramidal | 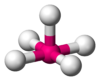 |
InCl52− in (NEt4)2InCl5 | |
| 6 | octahedral | 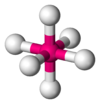 |
Fe(H2O)62+ | Na and Cl in NaCl |
| 6 | trigonal prismatic | Mo(SCHCHS)3 | As in NiAs, Mo in MoS2 Molybdenum disulfide Molybdenum disulfide is the inorganic compound with the formula MoS2. This black crystalline sulfide of molybdenum occurs as the mineral molybdenite. It is the principal ore from which molybdenum metal is extracted. The natural amorphous form is known as the rarer mineral jordisite. MoS2 is less... |
|
| 7 | pentagonal bipyramidal | 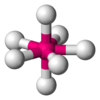 |
ZrF73− in (NH4)3ZrF7 | Pa in PaCl5 |
| 7 | face capped octahedral | [HoIII(PhCOCHCOPh)3(H2O)] | La in A-La2O3 Lanthanum(III) oxide Lanthanum oxide is La2O3, an inorganic compound containing the rare earth element lanthanum and oxygen. It is used to develop ferroelectric materials, and in optical materials.-Properties:... |
|
| 7 | trigonal prismatic, square face monocapped | TaF72− in K2TaF7 | ||
| 8 | cubic | Caesium chloride Caesium chloride Caesium chloride is the inorganic compound with the formula CsCl. This colorless solid is an important source of caesium ions in a variety of applications. Its crystal structure forms a major structural type where each caesium ion is coordinated by 8 chlorine ions... , calcium fluoride Calcium fluoride Calcium fluoride is the inorganic compound with the formula CaF2. This ionic compound of calcium and fluorine occurs naturally as the mineral fluorite . It is the source of most of the world's fluorine. This insoluble solid adopts a cubic structure wherein calcium is coordinated to eight fluoride... |
||
| 8 | square antiprism | TaF83− in Na3TaF8 | Thorium(IV) iodide Thorium(IV) iodide Thorium iodide is an inorganic chemical compound.... |
|
| 8 | dodecahedral (note whilst this is term generally used the correct term is bisdisphenoid or snub disphenoid Snub disphenoid In geometry, the snub disphenoid is one of the Johnson solids . It is a three-dimensional solid that has only equilateral triangles as faces, and is therefore a deltahedron. It is not a regular polyhedron because some vertices have four faces and others have five... as this polyhedron is a deltahedron Deltahedron A deltahedron is a polyhedron whose faces are all equilateral triangles. The name is taken from the Greek majuscule delta , which has the shape of an equilateral triangle. There are infinitely many deltahedra, but of these only eight are convex, having 4, 6, 8, 10, 12, 14, 16 and 20 faces... ) |
 |
Mo(CN)84− in K4[Mo(CN)8].2H2O | Zr in K2ZrF6 |
| 8 | hexagonal bipyramidal |  |
N in Li3N Lithium nitride Lithium nitride is a compound of lithium and nitrogen with the formula Li3N. It is the only stable alkali metal nitride... |
|
| 8 | octahedral, trans-bicapped | Ni in nickel arsenide, NiAs; 6 As neighbours + 2 Ni capping | ||
| 8 | trigonal prismatic, triangular face bicapped | Ca in CaFe2O4 | ||
| 8 | trigonal prismatic, square face bicapped | PuBr3 | ||
| 9 | trigonal prismatic, square face tricapped | 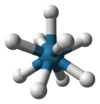 |
[ReH9]2− in potassium nonahydridorhenate Potassium nonahydridorhenate Potassium nonahydridorhenate is an inorganic compound with the formula K2ReH9. This colourless salt features the ReH92− anion, a rare example of a coordination complex bearing only hydride ligands.... |
SrCl2.6H2O, Th in RbTh3F13 |
| 9 | monocapped square antiprismatic | [Th(tropolonate)4(H2O)] | La in LaTe2 | |
| 10 | bicapped square antiprismatic | Th(C2O4)42− | ||
| 11 | Th in [ThIV(NO3)4(H2O)3] (NO3− is bidentate) | |||
| 12 | icosahedron |  |
Th in Th(NO3)62− ion in Mg[Th(NO3)6].8H2O | |
| 12 | cuboctahedron |  |
ZrIV(η3−(BH4)4) | atoms in fcc metals Periodic table (crystal structure) The structures of metallic elements adopted at standard temperatures and pressures are color coded and shown below, the only exception is mercury, Hg, which is a liquid and the structure refers to the low temperature form. The melting points of the metals is shown above the element symbol... e.g. Ca |
| 12 | anticuboctahedron, triangular orthobicupola |  |
atoms in hcp metals Periodic table (crystal structure) The structures of metallic elements adopted at standard temperatures and pressures are color coded and shown below, the only exception is mercury, Hg, which is a liquid and the structure refers to the low temperature form. The melting points of the metals is shown above the element symbol... e.g. Sc |
|
| 14 | bicapped hexagonal antiprismatic | U(BH4)4 |
Naming of inorganic compounds
IUPAC have introduced the polyhedral symbolPolyhedral symbol
The polyhedral symbol is sometimes used in coordination chemistry to indicate the approximate geometry of the coordinating atoms around the central atom. One or more italicised letters indicate the geometry, e.g. TP-3 which is followed by a number that gives the coordination number of the central...
as part of their IUPAC nomenclature of inorganic chemistry 2005 recommendations
IUPAC nomenclature of inorganic chemistry 2005
The Nomenclature of Inorganic Chemistry, IUPAC Recommendations 2005, commonly known as The Red Book, is a collection of rules for naming inorganic compounds, as recommended by the IUPAC.- Summary :...
to describe the geometry around an atom in a compound.
IUCr have proposed a symbol which is shown as a superscript in square brackets in the chemical formula. For example CaF2 would be Ca[8cb]F2[4t], where [8cb] means cubic coordination and [4t] means tetrahedral. The equivalent symbols in IUPAC are CU−8 and T−4 respectively.
The IUPAC symbol is applicable to complexes and molecules whereas the IUCr proposal applies to crystalline solids.

