
Coenzyme Q - cytochrome c reductase
Encyclopedia
In enzymology, a ubiquinol—cytochrome-c reductase is an enzyme
that catalyzes
the chemical reaction
Thus, the two substrates
of this enzyme are dihydroquinone (QH2) and ferri- (Fe3+) cytochrome c
, whereas its 3 products
are quinone
(Q), ferro- (Fe2+) cytochrome c, and H+
.
This enzyme belongs to the family of oxidoreductase
s, specifically those acting on diphenols and related substances as donor with a cytochrome as acceptor. This enzyme participates in oxidative phosphorylation
. It has four cofactors
: cytochrome C1
, cytochrome b-562, cytochrome b-566 and a 2-Iron ferredoxin
.
 The coenzyme Q : cytochrome c — oxidoreductase, sometimes called the cytochrome bc1 complex, and at other times complex III, is the third complex in the electron transport chain
The coenzyme Q : cytochrome c — oxidoreductase, sometimes called the cytochrome bc1 complex, and at other times complex III, is the third complex in the electron transport chain
, playing a critical role in biochemical generation of ATP (oxidative phosphorylation
). Complex III is a multisubunit transmembrane lipoprotein
encoded by both the mitochondrial (cytochrome b
) and the nuclear genomes (all other subunits). Complex III is present in the mitochondria of all animals and all aerobic eukaryotes and the inner membranes of most eubacteria. Mutations in Complex III cause exercise intolerance
as well as multisystem disorders.
has two b-type heme
s (bL and bH), the cytochrome c subunit has one c-type heme (c1
), and the Rieske Iron Sulfur Protein subunit (ISP) has a two iron, two sulfur iron-sulfur cluster
(2Fe•2S).
Structures of complex III: ,
by
oxidation of coenzyme Q
(CoQ) and the concomitant pumping of 4 protons from the mitochondrial matrix to the intermembrane space:
In the process called Q cycle
, two protons are consumed from the matrix (M), four protons are released into the inter membrane space (IM) and two electrons are passed to cytochrome c.
 The reaction mechanism for complex III (Cytochrome bc1 , Coenzyme Q: Cytochrome C Oxidoreductase) is known as the ubiquinone ("Q") cycle. In this cycle four protons get released into the Positive "P" side (inter membrane space), but only two protons get taken up from the Negative "N" side (matrix). As a result a proton gradient is formed across the membrane. In the overall reaction, two ubiquinol
The reaction mechanism for complex III (Cytochrome bc1 , Coenzyme Q: Cytochrome C Oxidoreductase) is known as the ubiquinone ("Q") cycle. In this cycle four protons get released into the Positive "P" side (inter membrane space), but only two protons get taken up from the Negative "N" side (matrix). As a result a proton gradient is formed across the membrane. In the overall reaction, two ubiquinol
s are oxidized to ubiquinones and one ubiquinone is reduced to ubiquinol
. In the complete mechanism, two electrons are transferred from ubiquinol to ubiquinone, via two cytochrome c intermediates.
Overall:
The reaction proceeds according to the following steps:
Round 1:
Round 2:
Some have been commercialized as fungicides (the strobilurin
derivatives, best known of which is azoxystrobin
; QoI
inhibitors) and as anti-malaria agents (atovaquone
).
results in the formation of superoxide
. The relevance of this otherwise minor side reaction is that superoxide
and other reactive oxygen species
are highly toxic and are thought to play a role in several pathologies, as well as aging (the free radical theory of aging). Electron leakage occurs mainly at the Qo site and is stimulated by antimycin A
. Antimycin A
locks the b hemes in the reduced state by preventing their re-oxidation at the Qi site, which, in turn, causes the steady-state concentrations of the Qo semiquinone to rise, the latter species reacting with oxygen
to form superoxide
. The effect of high membrane potential is thought to have a similar effect. Superoxide
produced at the Qo site can be released both into the mitochondrial matrix and into the intermembrane space (from where it can reach the cytosol. This could be explained by the fact that Complex III might produce superoxide
as membrane permeable HOO•
rather than as membrane impermeable O2-.
.
, a gene responsible for proper maturation of Complex III, can result in Björnstad syndrome
and the GRACILE syndrome
, which in neonates are lethal conditions that have multisystem and neurologic manifestations typifying severe mitochondrial disorders. The pathogenicity of several mutations has been verified in model systems such as yeast.
The extent to which these various pathologies are due to bioenergetic deficits or overeproduction of superoxide
is presently unknown.
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that catalyzes
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
- QH2 + 2 ferricytochrome c
 Q + 2 ferrocytochrome c + 2 H+
Q + 2 ferrocytochrome c + 2 H+
Thus, the two substrates
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
of this enzyme are dihydroquinone (QH2) and ferri- (Fe3+) cytochrome c
Cytochrome c
The Cytochrome complex, or cyt c is a small heme protein found loosely associated with the inner membrane of the mitochondrion. It belongs to the cytochrome c family of proteins. Cytochrome c is a highly soluble protein, unlike other cytochromes, with a solubility of about 100 g/L and is an...
, whereas its 3 products
Product (chemistry)
Product are formed during chemical reactions as reagents are consumed. Products have lower energy than the reagents and are produced during the reaction according to the second law of thermodynamics. The released energy comes from changes in chemical bonds between atoms in reagent molecules and...
are quinone
Quinone
A quinone is a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C– groups with any necessary rearrangement of double bonds," resulting in "a fully conjugated cyclic dione structure."...
(Q), ferro- (Fe2+) cytochrome c, and H+
Hydrogen ion
Hydrogen ion is recommended by IUPAC as a general term for all ions of hydrogen and its isotopes.Depending on the charge of the ion, two different classes can be distinguished: positively charged ions and negatively charged ions....
.
This enzyme belongs to the family of oxidoreductase
Oxidoreductase
In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule to another...
s, specifically those acting on diphenols and related substances as donor with a cytochrome as acceptor. This enzyme participates in oxidative phosphorylation
Oxidative phosphorylation
Oxidative phosphorylation is a metabolic pathway that uses energy released by the oxidation of nutrients to produce adenosine triphosphate . Although the many forms of life on earth use a range of different nutrients, almost all aerobic organisms carry out oxidative phosphorylation to produce ATP,...
. It has four cofactors
Cofactor (biochemistry)
A cofactor is a non-protein chemical compound that is bound to a protein and is required for the protein's biological activity. These proteins are commonly enzymes, and cofactors can be considered "helper molecules" that assist in biochemical transformations....
: cytochrome C1
Cytochrome C1
Cytochrome C1 is formed in the cytosol and targeted to the mitochondrial intermembrane space. It is one of the constituents of complex III, which forms the third proton pump in the mitochondrial electron transport chain....
, cytochrome b-562, cytochrome b-566 and a 2-Iron ferredoxin
Ferredoxin
Ferredoxins are iron-sulfur proteins that mediate electron transfer in a range of metabolic reactions. The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co...
.

Electron transport chain
An electron transport chain couples electron transfer between an electron donor and an electron acceptor with the transfer of H+ ions across a membrane. The resulting electrochemical proton gradient is used to generate chemical energy in the form of adenosine triphosphate...
, playing a critical role in biochemical generation of ATP (oxidative phosphorylation
Oxidative phosphorylation
Oxidative phosphorylation is a metabolic pathway that uses energy released by the oxidation of nutrients to produce adenosine triphosphate . Although the many forms of life on earth use a range of different nutrients, almost all aerobic organisms carry out oxidative phosphorylation to produce ATP,...
). Complex III is a multisubunit transmembrane lipoprotein
Lipoprotein
A lipoprotein is a biochemical assembly that contains both proteins and lipids water-bound to the proteins. Many enzymes, transporters, structural proteins, antigens, adhesins, and toxins are lipoproteins...
encoded by both the mitochondrial (cytochrome b
Cytochrome b
Cytochrome b/b6 is the main subunit of transmembrane cytochrome bc1 and b6f complexes. In addition, it commonly refers to a region of mtDNA used for population genetics and phylogenetics.- Function :...
) and the nuclear genomes (all other subunits). Complex III is present in the mitochondria of all animals and all aerobic eukaryotes and the inner membranes of most eubacteria. Mutations in Complex III cause exercise intolerance
Exercise intolerance
Exercise intolerance is a condition where the patient is unable to do physical exercise at the level or for the duration that would be expected of someone in his or her general physical condition, or experiences unusually severe post-exercise pain, fatigue, or other negative effects...
as well as multisystem disorders.
Nomenclature
The systematic name of this enzyme class is ubiquinol:ferricytochrome-c oxidoreductase. Other names in common use include:
|
|
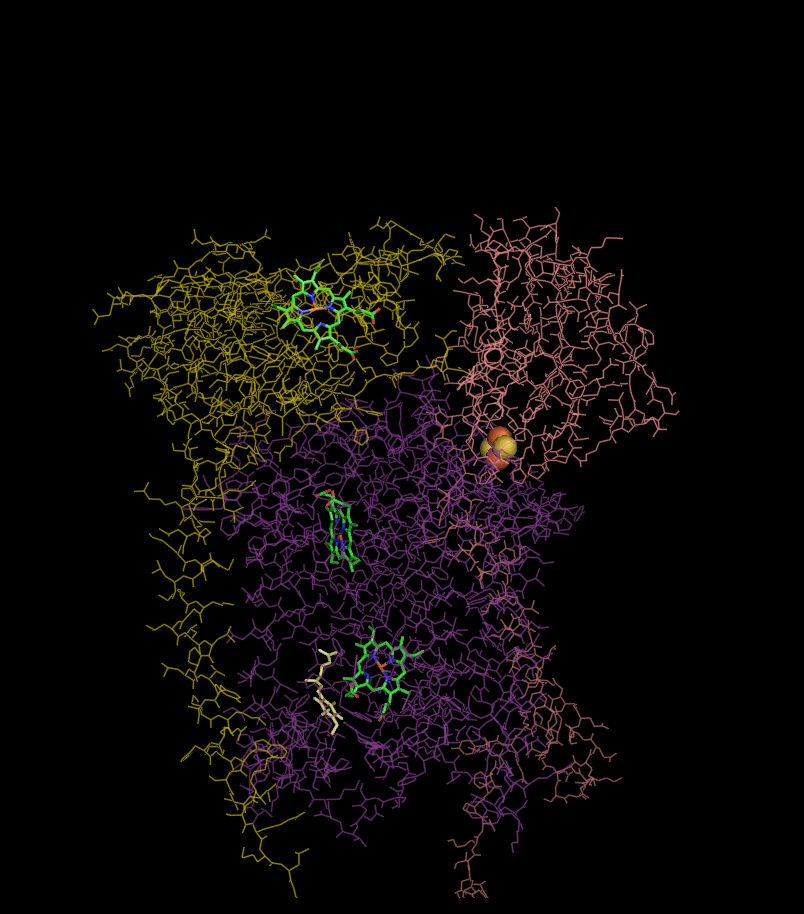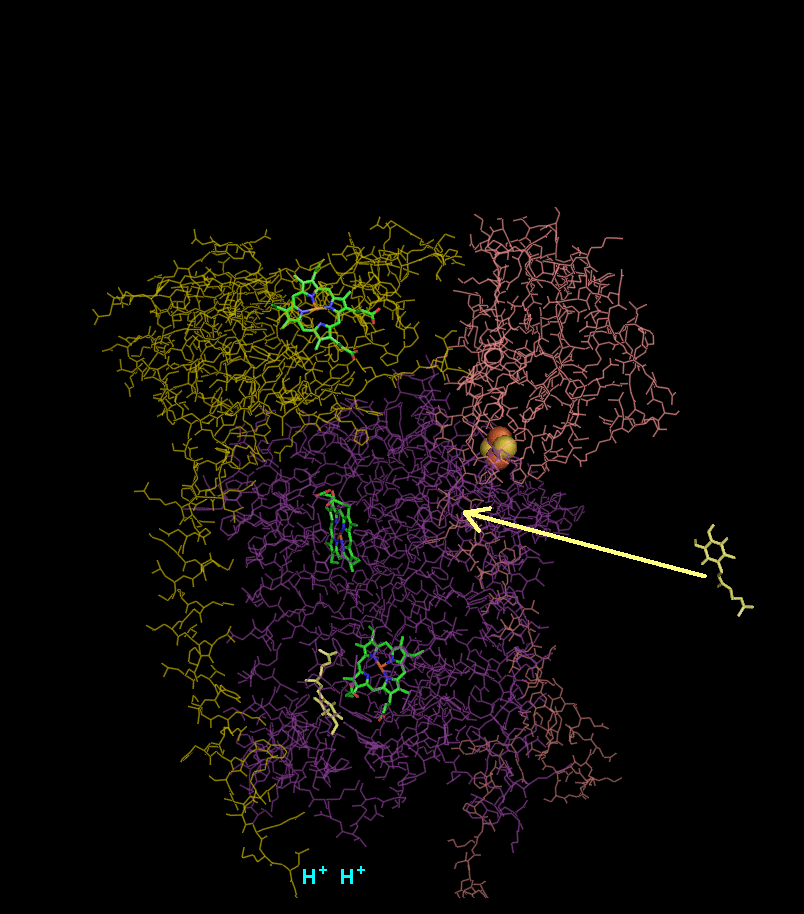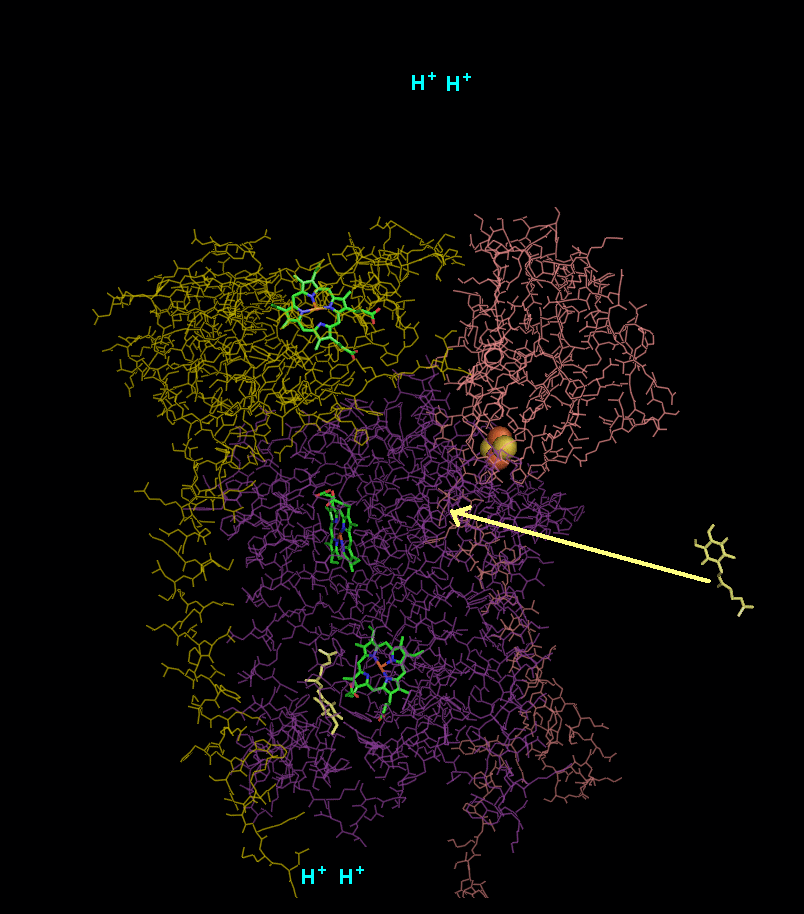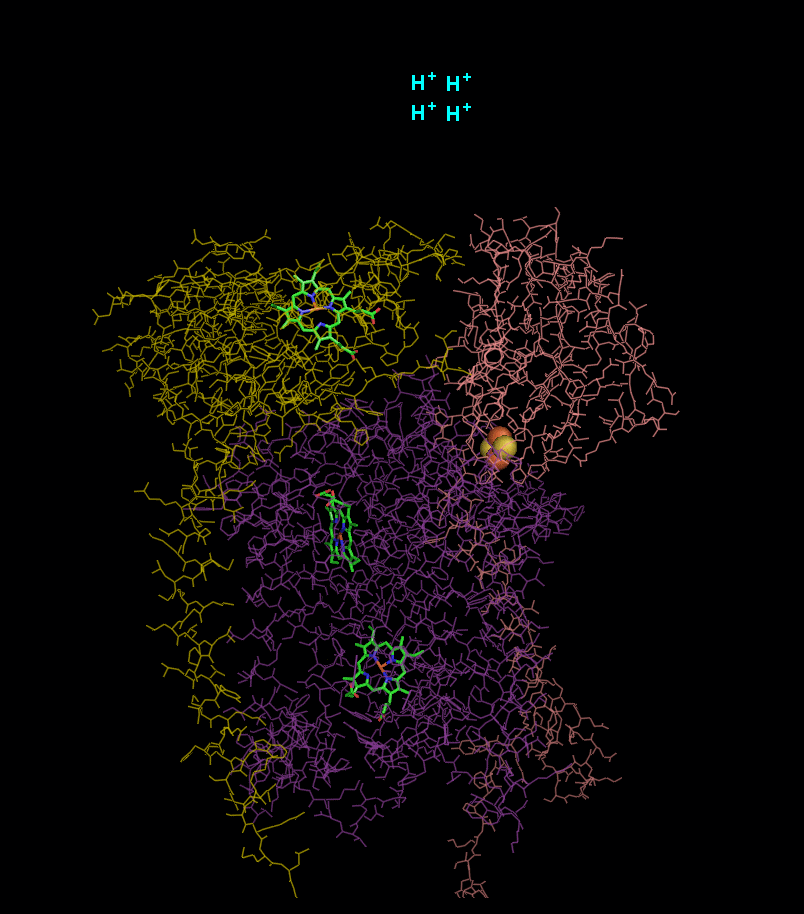
Structure
Compared to the other major proton-pumping subunits of the electron transport chain, the number of subunits found can be small, as small as three polypeptide chains. This number does increase, and eleven subunits are found in higher animals. Three subunits have prosthetic groups. The cytochrome b subunitCytochrome b
Cytochrome b/b6 is the main subunit of transmembrane cytochrome bc1 and b6f complexes. In addition, it commonly refers to a region of mtDNA used for population genetics and phylogenetics.- Function :...
has two b-type heme
Heme
A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
s (bL and bH), the cytochrome c subunit has one c-type heme (c1
Cytochrome C1
Cytochrome C1 is formed in the cytosol and targeted to the mitochondrial intermembrane space. It is one of the constituents of complex III, which forms the third proton pump in the mitochondrial electron transport chain....
), and the Rieske Iron Sulfur Protein subunit (ISP) has a two iron, two sulfur iron-sulfur cluster
Iron-sulfur cluster
For biological Fe-S clusters, see iron-sulfur proteins.Iron-sulfur clusters are ensembles of iron and sulfide centres. Fe-S clusters are most often discussed in the context of the biological role for iron-sulfur proteins. Many Fe-S clusters are known in the area of organometallic chemistry and as...
(2Fe•2S).
Structures of complex III: ,
Reaction
It catalyzes the reduction of cytochrome cCytochrome c
The Cytochrome complex, or cyt c is a small heme protein found loosely associated with the inner membrane of the mitochondrion. It belongs to the cytochrome c family of proteins. Cytochrome c is a highly soluble protein, unlike other cytochromes, with a solubility of about 100 g/L and is an...
by
oxidation of coenzyme Q
Coenzyme Q
Coenzyme Q10, also known as ubiquinone, ubidecarenone, coenzyme Q, and abbreviated at times to CoQ10 , CoQ, Q10, or Q, is a 1,4-benzoquinone, where Q refers to the quinone chemical group, and 10 refers to the number of isoprenyl chemical subunits in its tail.This oil-soluble, vitamin-like substance...
(CoQ) and the concomitant pumping of 4 protons from the mitochondrial matrix to the intermembrane space:
- QH2 + 2 cytochrome c (FeIII) + 2 H+in → Q + 2 cytochrome c (FeII) + 4 H+out
In the process called Q cycle
Q cycle
- History :The Q cycle describes a series of reactions first proposed by Peter Mitchell that describe how the sequential oxidation and reduction of the lipophilic electron carrier, ubiquinol-ubiquinone , can result in the net pumping of protons across a lipid bilayer...
, two protons are consumed from the matrix (M), four protons are released into the inter membrane space (IM) and two electrons are passed to cytochrome c.
Reaction Mechanism

Ubiquinol
Ubiquinol is an electron-rich form of coenzyme Q10.The natural ubiquinol form of coenzyme Q10 is 2,3-dimethoxy-5-methyl-6-poly prenyl-1,4-benzoquinol, where the polyprenylated side chain is 9-10 units long in mammals...
s are oxidized to ubiquinones and one ubiquinone is reduced to ubiquinol
Ubiquinol
Ubiquinol is an electron-rich form of coenzyme Q10.The natural ubiquinol form of coenzyme Q10 is 2,3-dimethoxy-5-methyl-6-poly prenyl-1,4-benzoquinol, where the polyprenylated side chain is 9-10 units long in mammals...
. In the complete mechanism, two electrons are transferred from ubiquinol to ubiquinone, via two cytochrome c intermediates.
Overall:
- 2 x QH2 oxidised to Q
- 1 x Q reduced to QH2
- 2 x Cyt c1 reduced
- 4 x H+ released into intermembrane space
- 2 x H+ picked up from matrix
The reaction proceeds according to the following steps:
Round 1:
- Cytochrome b binds a ubiquinol and a ubiquinone.
- The 2Fe/2S center and BL heme each pull an electron off the bound ubiquinol, releasing two hydrogens into the intermembrane space.
- One electron is transferred to cytochrome c1 from the 2Fe/2S centre, whilst another is transferred from the BL heme to the BH Heme.
- Cytochrome c1 transfers its electron to cytochrome cCytochrome cThe Cytochrome complex, or cyt c is a small heme protein found loosely associated with the inner membrane of the mitochondrion. It belongs to the cytochrome c family of proteins. Cytochrome c is a highly soluble protein, unlike other cytochromes, with a solubility of about 100 g/L and is an...
(not to be confused with cytochrome c1), and the BH Heme transfers its electron to a nearby ubiquinone, resulting in the formation of a ubisemiquinone. - Cytochrome c diffuses. The first ubiquinol (now oxidised to ubiquinone) is released, whilst the semiquinone remains bound.
Round 2:
- A second ubiquinol is bound by cytochrome b.
- The 2Fe/2S center and BL heme each pull an electron off the bound ubiquinol, releasing two hydrogens into the intermembrane space.
- One electron is transferred to cytochrome c1 from the 2Fe/2S centre, whilst another is transferred from the BL heme to the BH Heme.
- Cytocrome c1 then transfers its electron to cytochrome cCytochrome cThe Cytochrome complex, or cyt c is a small heme protein found loosely associated with the inner membrane of the mitochondrion. It belongs to the cytochrome c family of proteins. Cytochrome c is a highly soluble protein, unlike other cytochromes, with a solubility of about 100 g/L and is an...
, whilst the nearby semiquinone picks up a second electron from the BH Heme, along with two protons from the matrix. - The second ubiquinol (now oxidised to ubiquinone), along with the newly formed ubiquinol are released.
Inhibitors of complex III
There are three distinct groups of Complex III inhibitors.- Antimycin A binds to the Qi site and inhibits the transfer of electrons in Complex III from heme bH to oxidized Q (Qi site inhibitor).
- MyxothiazolMyxothiazolMyxothiazol is an inhibitor of the mitochondrial cytochrome bc1 complex ....
and stigmatellinStigmatellinStigmatellin is a potent inhibitor of the quinol oxidation site of the cytochrome bc1 complex in mitochondria and the cytochrome b6f complex of thylakoid membranes....
binds to the Qo site and inhibits the transfer of electrons from reduced QH2 to the Rieske Iron sulfur protein. Myxothiazol and stigmatellin bind to distinct pockets within the Qo site.- Myxothiazol binds very close to cytochrome bL (hence termed a "proximal" inhibitor).
- Stigmatellin binds near the Rieske Iron sulfur protein, with which it strongly interacts.
Some have been commercialized as fungicides (the strobilurin
Strobilurin
Strobilurins are a group of chemical compounds used in agriculture as fungicides. They are part of the larger group of QoI inhibitors, which act to inhibit the respiratory chain at the level of Complex III....
derivatives, best known of which is azoxystrobin
Azoxystrobin
Azoxystrobin is a fungicide commonly used in agriculture. The substance is used as an active agent protecting plants and fruit/vegetables from fungal diseases.-Origin:...
; QoI
QoI
Qo inhibitors , or Quinone outside inhibitors are a group of fungicides used in agriculture. They represent the most important development made in fungicides by the chemicals industry...
inhibitors) and as anti-malaria agents (atovaquone
Atovaquone
Atovaquone is a chemical compound that belongs to the class of naphthalenes. Atovaquone is a hydroxy-1,4-naphthoquinone, an analog of ubiquinone, with antipneumocystic activity. Its average wholesale price is about US$2.13 per standard 250 mg. tablet...
).
Oxygen free radicals
A small fraction of electrons leave the electron transport chain before reaching complex IV. Premature electron leakage to oxygenOxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
results in the formation of superoxide
Superoxide
A superoxide, also known by the obsolete name hyperoxide, is a compound that possesses the superoxide anion with the chemical formula O2−. The systematic name of the anion is dioxide. It is important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature...
. The relevance of this otherwise minor side reaction is that superoxide
Superoxide
A superoxide, also known by the obsolete name hyperoxide, is a compound that possesses the superoxide anion with the chemical formula O2−. The systematic name of the anion is dioxide. It is important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature...
and other reactive oxygen species
Reactive oxygen species
Reactive oxygen species are chemically reactive molecules containing oxygen. Examples include oxygen ions and peroxides. Reactive oxygen species are highly reactive due to the presence of unpaired valence shell electrons....
are highly toxic and are thought to play a role in several pathologies, as well as aging (the free radical theory of aging). Electron leakage occurs mainly at the Qo site and is stimulated by antimycin A
Antimycin A
Antimycins are a group of secondary metabolites produced by Streptomyces bacteria.-Uses:It is the active ingredient in Fintrol, a chemical piscicide used in fisheries management and in the catfish industry.-Mechanism of action:...
. Antimycin A
Antimycin A
Antimycins are a group of secondary metabolites produced by Streptomyces bacteria.-Uses:It is the active ingredient in Fintrol, a chemical piscicide used in fisheries management and in the catfish industry.-Mechanism of action:...
locks the b hemes in the reduced state by preventing their re-oxidation at the Qi site, which, in turn, causes the steady-state concentrations of the Qo semiquinone to rise, the latter species reacting with oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
to form superoxide
Superoxide
A superoxide, also known by the obsolete name hyperoxide, is a compound that possesses the superoxide anion with the chemical formula O2−. The systematic name of the anion is dioxide. It is important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature...
. The effect of high membrane potential is thought to have a similar effect. Superoxide
Superoxide
A superoxide, also known by the obsolete name hyperoxide, is a compound that possesses the superoxide anion with the chemical formula O2−. The systematic name of the anion is dioxide. It is important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature...
produced at the Qo site can be released both into the mitochondrial matrix and into the intermembrane space (from where it can reach the cytosol. This could be explained by the fact that Complex III might produce superoxide
Superoxide
A superoxide, also known by the obsolete name hyperoxide, is a compound that possesses the superoxide anion with the chemical formula O2−. The systematic name of the anion is dioxide. It is important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature...
as membrane permeable HOO•
Hydroperoxyl
The hydroperoxyl radical, also known as the perhydroxyl radical, is the protonated form of superoxide with the chemical formula HO2.-Reactivity:The superoxide anion, O2−, and the hydroperoxyl radical are in equilibrium in aqueous solution:...
rather than as membrane impermeable O2-.
Superoxide
A superoxide, also known by the obsolete name hyperoxide, is a compound that possesses the superoxide anion with the chemical formula O2−. The systematic name of the anion is dioxide. It is important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature...
.
Mutations in Complex III genes in human disease
Mutations in Complex III-related genes typically manifest as exercise intolerance. Other mutations have been reported to cause septo-optic displaisa and multisystem disorders. However, mutations in BCS1LBCS1L
-Further reading:...
, a gene responsible for proper maturation of Complex III, can result in Björnstad syndrome
Björnstad syndrome
Björnstad syndrome is a congenital condition involving deafness and hair abnormalities.It was first characterized in 1965, in Oslo, by prof. Roar Theodor Bjørnstad .It has been mapped to BCS1L....
and the GRACILE syndrome
GRACILE syndrome
GRACILE syndrome is an autosomal recessive genetic disorder, one of the Finnish heritage diseases. It is caused by mutation in BCS1L gene.GRACILE is an acronym for growth retardation, amino aciduria, cholestasis, iron overload, lactic acidosis, and early death...
, which in neonates are lethal conditions that have multisystem and neurologic manifestations typifying severe mitochondrial disorders. The pathogenicity of several mutations has been verified in model systems such as yeast.
The extent to which these various pathologies are due to bioenergetic deficits or overeproduction of superoxide
Superoxide
A superoxide, also known by the obsolete name hyperoxide, is a compound that possesses the superoxide anion with the chemical formula O2−. The systematic name of the anion is dioxide. It is important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature...
is presently unknown.
External links
- cytochrome bc1 complex site (Edward A. Berry) at lbl.gov
- cytochrome bc1 complex site (Antony R. Crofts) at uiuc.edu
- PROMISE Database: cytochrome bc1 complex at scripps.edu
- Interactive Molecular Model of Complex III (Requires MDL Chime) - Calculated positions of bc1 and related complexes in membranes

