Chymotrypsin
Encyclopedia
Chymotrypsin is a digestive enzyme
that can perform proteolysis
. Chymotrypsin preferentially cleaves peptide amide bonds where the carboxyl side of the amide bond (the P1 position) is a tyrosine
, tryptophan
, or phenylalanine
. These amino acid
s contain an aromatic ring in their sidechain
that fits into a 'hydrophobic
pocket' (the S1 position) of the enzyme. The hydrophobic and shape complementarity between the peptide substrate P1 sidechain and the enzyme S1 binding cavity accounts for the substrate specificity of this enzyme. Chymotrypsin also hydrolyzes other amide bonds in peptides at slower rates, particularly those containing leucine
at the P1 position.
by protein biosynthesis
as a precursor called chymotrypsinogen
that is enzymatically inactive. On cleavage by trypsin
into two parts that are still connected via an S-S bond, cleaved chymotrypsinogen molecules can activate each other by removing two small peptides in a trans-proteolysis. The resulting molecule is active chymotrypsin, a three-polypeptide
molecule interconnected via disulfide bond
s.
reaction, which despite being thermodynamically favourable occurs extremely slowly in the absence of a catalyst. The main substrates of chymotrypsin include tryptophan
, tyrosine
, phenylalanine
, leucine
, and methionine
, which are cleaved at the carboxyl terminal. Like many proteases, chymotrypsin will also hydrolyse amide bonds in vitro, a virtue that enabled the use of substrate analogs such as N-acetyl-L-phenylalanine p-nitrophenyl amide for enzyme assays.
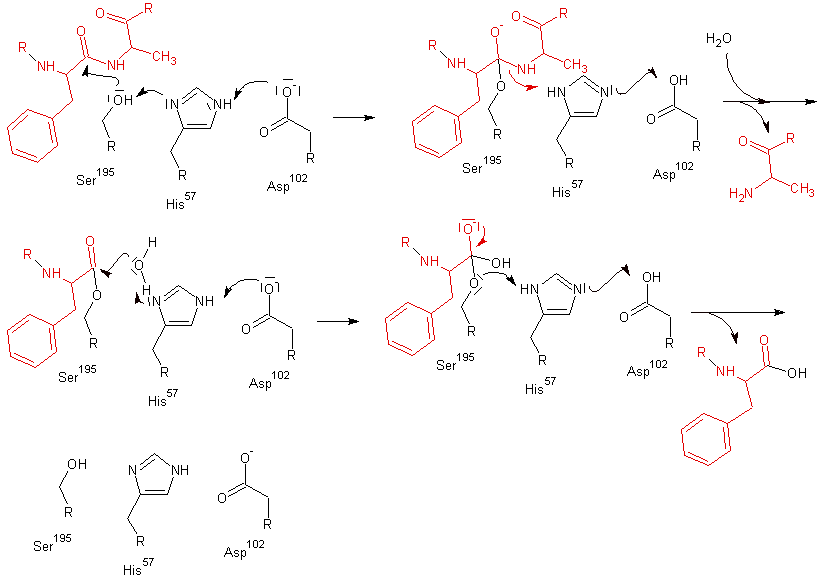
Chymotrypsin cleaves peptide bonds by attacking the unreactive carbonyl group with a powerful nucleophile, the serine 195 residue
located in the active site of the enzyme, which briefly becomes covalently bonded to the substrate, forming an enzyme-substrate intermediate.
These findings rely on inhibition assays and the study of the kinetics of cleavage of the aforementioned substrate, exploiting the fact that the enzyme-substrate intermediate p-nitrophenolate
has a yellow colour, enabling us to measure its concentration by measuring light absorbance at 410 nm.
It was found that the reaction of chymotrypsin with its substrate takes place in two stages, an initial “burst” phase at the beginning of the reaction and a steady-state phase following Michaelis-Menten kinetics
. It is also called "ping-pong" mechanism. The mode of action of chymotrypsin explains this as hydrolysis takes place in two steps. First acylation of the substrate to form an acyl-enzyme intermediate and then deacylation in order to return the enzyme to its original state.
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that can perform proteolysis
Proteolysis
Proteolysis is the directed degradation of proteins by cellular enzymes called proteases or by intramolecular digestion.-Purposes:Proteolysis is used by the cell for several purposes...
. Chymotrypsin preferentially cleaves peptide amide bonds where the carboxyl side of the amide bond (the P1 position) is a tyrosine
Tyrosine
Tyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
, tryptophan
Tryptophan
Tryptophan is one of the 20 standard amino acids, as well as an essential amino acid in the human diet. It is encoded in the standard genetic code as the codon UGG...
, or phenylalanine
Phenylalanine
Phenylalanine is an α-amino acid with the formula C6H5CH2CHCOOH. This essential amino acid is classified as nonpolar because of the hydrophobic nature of the benzyl side chain. L-Phenylalanine is an electrically neutral amino acid, one of the twenty common amino acids used to biochemically form...
. These amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s contain an aromatic ring in their sidechain
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
that fits into a 'hydrophobic
Hydrophobe
In chemistry, hydrophobicity is the physical property of a molecule that is repelled from a mass of water....
pocket' (the S1 position) of the enzyme. The hydrophobic and shape complementarity between the peptide substrate P1 sidechain and the enzyme S1 binding cavity accounts for the substrate specificity of this enzyme. Chymotrypsin also hydrolyzes other amide bonds in peptides at slower rates, particularly those containing leucine
Leucine
Leucine is a branched-chain α-amino acid with the chemical formula HO2CCHCH2CH2. Leucine is classified as a hydrophobic amino acid due to its aliphatic isobutyl side chain. It is encoded by six codons and is a major component of the subunits in ferritin, astacin and other 'buffer' proteins...
at the P1 position.
Activating chymotrypsinogen into chymotrypsin
Chymotrypsin is synthesized in the pancreasPancreas
The pancreas is a gland organ in the digestive and endocrine system of vertebrates. It is both an endocrine gland producing several important hormones, including insulin, glucagon, and somatostatin, as well as a digestive organ, secreting pancreatic juice containing digestive enzymes that assist...
by protein biosynthesis
Protein biosynthesis
Protein biosynthesis is the process in which cells build or manufacture proteins. The term is sometimes used to refer only to protein translation but more often it refers to a multi-step process, beginning with amino acid synthesis and transcription of nuclear DNA into messenger RNA, which is then...
as a precursor called chymotrypsinogen
Chymotrypsinogen
Chymotrypsinogen is a precursor of the digestive enzyme chymotrypsin.This molecule is inactive and must be cleaved by trypsin, and then by other...
that is enzymatically inactive. On cleavage by trypsin
Trypsin
Trypsin is a serine protease found in the digestive system of many vertebrates, where it hydrolyses proteins. Trypsin is produced in the pancreas as the inactive proenzyme trypsinogen. Trypsin cleaves peptide chains mainly at the carboxyl side of the amino acids lysine or arginine, except when...
into two parts that are still connected via an S-S bond, cleaved chymotrypsinogen molecules can activate each other by removing two small peptides in a trans-proteolysis. The resulting molecule is active chymotrypsin, a three-polypeptide
Peptide
Peptides are short polymers of amino acid monomers linked by peptide bonds. They are distinguished from proteins on the basis of size, typically containing less than 50 monomer units. The shortest peptides are dipeptides, consisting of two amino acids joined by a single peptide bond...
molecule interconnected via disulfide bond
Disulfide bond
In chemistry, a disulfide bond is a covalent bond, usually derived by the coupling of two thiol groups. The linkage is also called an SS-bond or disulfide bridge. The overall connectivity is therefore R-S-S-R. The terminology is widely used in biochemistry...
s.
Action and kinetics of chymotrypsin
In vivo, chymotrypsin is a proteolytic enzyme (Serine protease) acting in the digestive systems of mammals and other organisms. It facilitates the cleavage of peptide bonds by a hydrolysisHydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
reaction, which despite being thermodynamically favourable occurs extremely slowly in the absence of a catalyst. The main substrates of chymotrypsin include tryptophan
Tryptophan
Tryptophan is one of the 20 standard amino acids, as well as an essential amino acid in the human diet. It is encoded in the standard genetic code as the codon UGG...
, tyrosine
Tyrosine
Tyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
, phenylalanine
Phenylalanine
Phenylalanine is an α-amino acid with the formula C6H5CH2CHCOOH. This essential amino acid is classified as nonpolar because of the hydrophobic nature of the benzyl side chain. L-Phenylalanine is an electrically neutral amino acid, one of the twenty common amino acids used to biochemically form...
, leucine
Leucine
Leucine is a branched-chain α-amino acid with the chemical formula HO2CCHCH2CH2. Leucine is classified as a hydrophobic amino acid due to its aliphatic isobutyl side chain. It is encoded by six codons and is a major component of the subunits in ferritin, astacin and other 'buffer' proteins...
, and methionine
Methionine
Methionine is an α-amino acid with the chemical formula HO2CCHCH2CH2SCH3. This essential amino acid is classified as nonpolar. This amino-acid is coded by the codon AUG, also known as the initiation codon, since it indicates mRNA's coding region where translation into protein...
, which are cleaved at the carboxyl terminal. Like many proteases, chymotrypsin will also hydrolyse amide bonds in vitro, a virtue that enabled the use of substrate analogs such as N-acetyl-L-phenylalanine p-nitrophenyl amide for enzyme assays.

Chymotrypsin cleaves peptide bonds by attacking the unreactive carbonyl group with a powerful nucleophile, the serine 195 residue
Sequence of proteases - chymotrypsin A - trypsin - elastase
In the catalytic triad of chymotrypsin, trypsin, and elastase, the three proteases function at three important molecular 'cutting' points. The sites are at amino acid residues of: histidine 57, aspartic acid 102, and serine 195....
located in the active site of the enzyme, which briefly becomes covalently bonded to the substrate, forming an enzyme-substrate intermediate.
These findings rely on inhibition assays and the study of the kinetics of cleavage of the aforementioned substrate, exploiting the fact that the enzyme-substrate intermediate p-nitrophenolate
4-Nitrophenol
4-Nitrophenol is a phenolic compound that has a nitro group at the opposite position of hydroxy group on the benzene ring.-Properties:...
has a yellow colour, enabling us to measure its concentration by measuring light absorbance at 410 nm.
It was found that the reaction of chymotrypsin with its substrate takes place in two stages, an initial “burst” phase at the beginning of the reaction and a steady-state phase following Michaelis-Menten kinetics
Michaelis-Menten kinetics
In biochemistry, Michaelis–Menten kinetics is one of the simplest and best-known models of enzyme kinetics. It is named after German biochemist Leonor Michaelis and Canadian physician Maud Menten. The model takes the form of an equation describing the rate of enzymatic reactions, by relating...
. It is also called "ping-pong" mechanism. The mode of action of chymotrypsin explains this as hydrolysis takes place in two steps. First acylation of the substrate to form an acyl-enzyme intermediate and then deacylation in order to return the enzyme to its original state.
Isozymes
External links
- The MEROPSMeropsMerops may refer to:* Merops , a genus of bee-eaters.* MEROPS, an on-line database for peptidases.It may also refer to several figures from Greek mythology:* King of Ethiopia, husband of Clymene, who lay with Helios and bore Phaethon...
online database for peptidases and their inhibitors: S01.001

