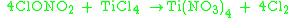Chlorine nitrate
Encyclopedia
Chlorine nitrate is an important atmospheric gas present in the stratosphere
. It is an important sink of chlorine that contributes to the depletion of ozone.
It explosively reacts with metals, metal chlorides, alcohols, ethers, and most organic materials. When it is heated to decomposition, it emits toxic fumes of Cl2 and NOx.
It can be produced by the reaction of dichlorine monoxide
and dinitrogen pentoxide
at 0°C: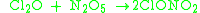
It can also react with alkene
s: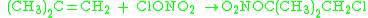
Chlorine nitrate reacts with metal chloride
s: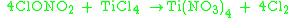
Stratosphere
The stratosphere is the second major layer of Earth's atmosphere, just above the troposphere, and below the mesosphere. It is stratified in temperature, with warmer layers higher up and cooler layers farther down. This is in contrast to the troposphere near the Earth's surface, which is cooler...
. It is an important sink of chlorine that contributes to the depletion of ozone.
It explosively reacts with metals, metal chlorides, alcohols, ethers, and most organic materials. When it is heated to decomposition, it emits toxic fumes of Cl2 and NOx.
It can be produced by the reaction of dichlorine monoxide
Dichlorine monoxide
Dichlorine monoxide, Cl2O, also known as, oxygen dichloride, dichlorine oxide or chlorine oxide is a chlorine oxide. It is a brownish yellow gas at room temperature which can explode in high concentrations when heated or sparked....
and dinitrogen pentoxide
Dinitrogen pentoxide
Dinitrogen pentoxide is the chemical compound with the formula N2O5. Also known as nitrogen pentoxide, N2O5 is one of the binary nitrogen oxides, a family of compounds that only contain nitrogen and oxygen...
at 0°C:
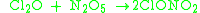
It can also react with alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s:
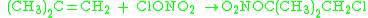
Chlorine nitrate reacts with metal chloride
Chloride
The chloride ion is formed when the element chlorine, a halogen, picks up one electron to form an anion Cl−. The salts of hydrochloric acid HCl contain chloride ions and can also be called chlorides. The chloride ion, and its salts such as sodium chloride, are very soluble in water...
s: