Chiral synthesis
Encyclopedia
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
that introduces one or more new and desired elements of chirality
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
. This methodology is important in the field of pharmaceuticals because the different enantiomer
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
s or diastereomer
Diastereomer
Diastereomers are stereoisomers that are not enantiomers.Diastereomerism occurs when two or more stereoisomers of a compound have different configurations at one or more of the equivalent stereocenters and are not mirror images of each other.When two diastereoisomers differ from each other at...
s of a molecule often have different biological activity.
Approaches
There are three main approaches to enantioselective synthesis:- chiral pool synthesisChiral pool synthesisChiral pool synthesis is a strategy that aims to improve the efficiency of chiral synthesis. It starts the organic synthesis of a complex enantiopure chemical compound from a stock of readily available enantiopure substances. Common chiral starting materials include monosaccharides and amino acids...
- chiral auxiliariesChiral auxiliaryA chiral auxiliary is a chemical compound or unit that is temporarily incorporated into an organic synthesis so that it can be carried out asymmetrically with the selective formation of one of two enantiomers...
- enantioselective catalysis
It is often advantageous to combine two or all of these approaches.
Chiral pool synthesis
Chiral pool synthesisChiral pool synthesis
Chiral pool synthesis is a strategy that aims to improve the efficiency of chiral synthesis. It starts the organic synthesis of a complex enantiopure chemical compound from a stock of readily available enantiopure substances. Common chiral starting materials include monosaccharides and amino acids...
is the easiest approach: A chiral starting material is manipulated through successive reactions using achiral reagents that retain its chirality to obtain the desired target molecule. This is especially attractive for target molecules having the similar chirality to a relatively inexpensive naturally occurring building-block such as a sugar
Sugar
Sugar is a class of edible crystalline carbohydrates, mainly sucrose, lactose, and fructose, characterized by a sweet flavor.Sucrose in its refined form primarily comes from sugar cane and sugar beet...
or amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
. However, the number of possible reactions the molecule can undergo is restricted, and tortuous synthetic routes may be required. Also, this approach requires a stoichiometric amount of the enantiopure starting material, which may be rather expensive if not occurring in nature, whereas chiral catalysis requires only a catalytic amount of chiral material.
Enantioselective induction
What many strategies in chiral synthesis have in common is asymmetric inductionAsymmetric induction
Asymmetric induction in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment...
. The aim is to make enantiomer
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
s into diastereomer
Diastereomer
Diastereomers are stereoisomers that are not enantiomers.Diastereomerism occurs when two or more stereoisomers of a compound have different configurations at one or more of the equivalent stereocenters and are not mirror images of each other.When two diastereoisomers differ from each other at...
s, since diastereomers have different reactivity, but enantiomers do not. To make enantiomers into diastereomers, the reagents or the catalyst need to be incorporated with an enantiopure chiral center. The reaction will now proceed differently for different enantiomers, because the transition state of the reaction can exist in two diastereomers with respect to the enantiopure center, and these diastereomers react differently.
Enantioselective induction can also occur intramolecularly when given a chiral starting material. This enantioinduction can be exploited, especially when the goal is to make several consecutive chiral centers to give a specific enantiomer of a specific diastereomer. An aldol reaction
Aldol reaction
The aldol reaction is a powerful means of forming carbon–carbon bonds in organic chemistry.Discovered independently by Charles-Adolphe Wurtz and Alexander Porfyrevich Borodin in 1872, the reaction combines two carbonyl compounds to form a new β-hydroxy carbonyl compound...
, for example, is inherently diastereoselective; if the aldehyde is enantiopure, the resulting aldol adduct is diastereomerically and enantiomerically pure.
Chiral auxiliary
One enantioinduction strategy is the use of a chiral auxiliaryChiral auxiliary
A chiral auxiliary is a chemical compound or unit that is temporarily incorporated into an organic synthesis so that it can be carried out asymmetrically with the selective formation of one of two enantiomers...
, which forms an adduct to the starting materials and physically blocks the other trajectory for attack, leaving only the desired trajectory open. Assuming the chiral auxiliary is enantiopure, the different trajectories are not equivalent, but diastereomeric. The auxiliary shares problems similar to protecting group
Protecting group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group in order to obtain chemoselectivity in a subsequent chemical reaction...
s; like protecting groups, auxiliaries require a reaction step to add and another to remove, increasing cost and decreasing yield.
Enantioselective catalysis
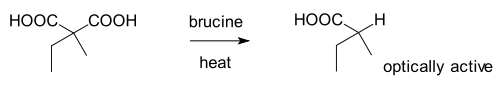
The oldest enantioselective synthesis is the enantioselective decarboxylationDecarboxylation
Decarboxylation is a chemical reaction that releases carbon dioxide . Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carbonation, the addition of CO2 to...
of the malonic acid
Malonic acid
Malonic acid is a dicarboxylic acid with structure CH22. The ionised form of malonic acid, as well as its esters and salts, are known as malonates. For example, diethyl malonate is malonic acid's ethyl ester...
2-ethyl-2-methylmalonic acid mediated by brucine
Brucine
Brucine is a bitter alkaloid closely related to strychnine. It occurs in several plant species, the most well known being the Strychnos nux-vomica tree, found in South-East Asia.While brucine is related to strychnine, it is not as poisonous...
(forming the salt) as reported by Willy Marckwald in 1904:
Small amounts of chiral, enantiomerically pure (or enriched) catalysts promote reactions and lead to the formation of large amounts of enantiomerically pure or enriched products. Mostly, three different kinds of chiral catalysts are employed:
- metal ligand complexes derived from chiral ligandChiral ligandIn chemistry a chiral ligand is a specially adapted ligand used for asymmetric synthesis. This ligand is an enantiopure organic compound which combines with a metal center by chelation to form an asymmetric catalyst. This catalyst engages in a chemical reaction and transfers its chirality to the...
s - chiral organocatalysts
- biocatalysts.
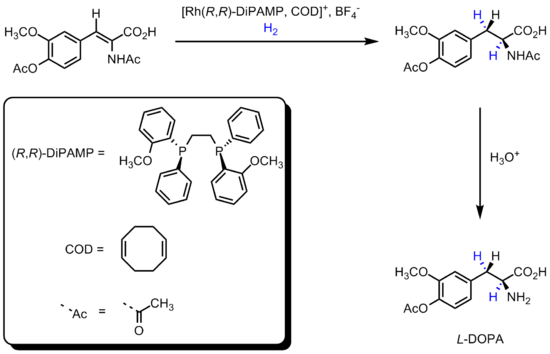
The first methods were pioneered by William S. Knowles and Ryōji Noyori
Ryoji Noyori
is a Japanese chemist. He won the Nobel Prize in Chemistry in 2001. Noyori shared half of the prize with William S. Knowles for the study of chirally catalyzed hydrogenations; the second half of the Prize went to K. Barry Sharpless for his study in chirally catalyzed oxidation reactions...
(Nobel Prize in Chemistry
Nobel Prize in Chemistry
The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outstanding contributions in chemistry, physics, literature,...
2001). Knowles in 1968 replaced the achiral triphenylphosphine
Triphenylphosphine
Triphenylphosphine is a common organophosphorus compound with the formula P3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature...
ligands in Wilkinson's catalyst
Wilkinson's catalyst
Wilkinson's catalyst is the common name for chlorotrisrhodium, a coordination compound with the formula RhCl3 . It is named after the late organometallic chemist and 1973 Nobel Laureate, Sir Geoffrey Wilkinson who popularized its use.-Structure and basic properties:The compound is a square planar,...
by the chiral phosphine ligands P(Ph)(Me)(Propyl), thus creating the first asymmetric catalyst. This experimental catalyst was employed in an asymmetric hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
with a modest 15% enantiomeric excess
Enantiomeric excess
The enantiomeric excess of a substance is a measure of how pure it is. In this case, the impurity is the undesired enantiomer .-Definition:...
result. The methodology was ultimately used by him (while working for the Monsanto Company company) in an enantioselective hydrogenation step in the industrial production of L-DOPA:
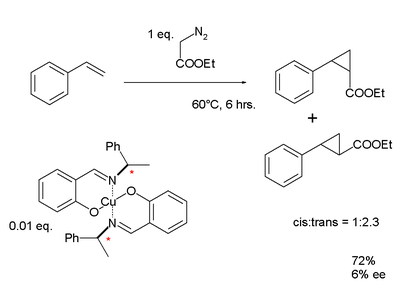
In the same year and independently, Noyori published his chiral ligand for a cyclopropanation reaction of styrene. In common with Knowles' findings, Noyori's results for the enantiomeric excess for this first-generation ligand was disappointingly low: 6%.
Examples of enantioselective catalysis include:
- BINAPBINAPBINAP is an abbreviation for the organophosphorus compound 2,2'-bis-1,1'-binaphthyl. This chiral ligand is widely used in asymmetric synthesis. It consists of a pair of 2-diphenylphosphinonaphthyl groups linked at the 1 and 1´ positions. This C2-symmetric framework lacks stereogenic atom, but...
, a chiral phosphinePhosphinePhosphine is the compound with the chemical formula PH3. It is a colorless, flammable, toxic gas. Pure phosphine is odourless, but technical grade samples have a highly unpleasant odor like garlic or rotting fish, due to the presence of substituted phosphine and diphosphine...
, used in combination with compounds of rutheniumRutheniumRuthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
or rhodiumRhodiumRhodium is a chemical element that is a rare, silvery-white, hard and chemically inert transition metal and a member of the platinum group. It has the chemical symbol Rh and atomic number 45. It is composed of only one isotope, 103Rh. Naturally occurring rhodium is found as the free metal, alloyed...
. These complexesComplex (chemistry)In chemistry, a coordination complex or metal complex, is an atom or ion , bonded to a surrounding array of molecules or anions, that are in turn known as ligands or complexing agents...
catalyse the hydrogenationHydrogenationHydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
of functionalised alkeneAlkeneIn organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s well on only one face of the molecule. This process also developed by Ryōji NoyoriRyoji Noyoriis a Japanese chemist. He won the Nobel Prize in Chemistry in 2001. Noyori shared half of the prize with William S. Knowles for the study of chirally catalyzed hydrogenations; the second half of the Prize went to K. Barry Sharpless for his study in chirally catalyzed oxidation reactions...
is commercialized as the industrial synthesis of mentholMentholMenthol is an organic compound made synthetically or obtained from peppermint or other mint oils. It is a waxy, crystalline substance, clear or white in color, which is solid at room temperature and melts slightly above. The main form of menthol occurring in nature is -menthol, which is assigned...
using a chiral BINAP-rhodiumRhodiumRhodium is a chemical element that is a rare, silvery-white, hard and chemically inert transition metal and a member of the platinum group. It has the chemical symbol Rh and atomic number 45. It is composed of only one isotope, 103Rh. Naturally occurring rhodium is found as the free metal, alloyed...
complex. - The other part of that Nobel prize concerned the Sharpless bishydroxylation
- NaproxenNaproxenNaproxen sodium is a nonsteroidal anti-inflammatory drug commonly used for the reduction of pain, fever, inflammation and stiffness caused by conditions such as:...
is synthesized with a chiral phosphine ligand in a hydrocyanationHydrocyanationHydrocyanation is, most fundamentally, the process whereby H+ and –CN ions are added to a molecular substrate. Usually the substrate is an alkene and the product is a nitrile. When –CN is a ligand in a transition metal complex, its basicity makes it difficult to dislodge, so, in this...
reaction - asymmetric catalytic reductionAsymmetric catalytic reductionAsymmetric catalytic reduction is the use of various chiral catalysts to reduce a prochiral organic compound to obtain a chiral product. This is one of the several techniques used in chiral synthesis....
and oxidationAsymmetric catalytic oxidationAsymmetric catalytic oxidation is a technique of oxidizing various substrates to give an enantiopure product using a catalyst.-Reactions:*Jacobsen epoxidation of alkenes using manganese-salen complex and NaOCl...
Biocatalysis & organocatalysis
BiocatalysisBiocatalysis
Biocatalysis is the use of natural catalysts, such as protein enzymes, to perform chemical transformations on organic compounds. Both enzymes that have been more or less isolated and enzymes still residing inside living cells are employed for this task....
makes use of enzymes to effect chemical reagents stereoselectively. Some small organic molecules can also be used to help accelerate the desired reaction; this method is known as organocatalysis
Organocatalysis
In organic chemistry, the term Organocatalysis refers to a form of catalysis, whereby the rate of a chemical reaction is increased by an organic catalyst referred to as an "organocatalyst" consisting of carbon, hydrogen, sulfur and other nonmetal elements found in organic compounds...
. If the organic molecule is chiral, it may react preferentially with a particular enantiomer or diastereomer of substrate.
Alternatives
Apart from enantioselective synthesis, racemic mixtures of compounds can be resolved by various techniques in chiral resolutionChiral resolution
Chiral resolution in stereochemistry is a process for the separation of racemic compounds into their enantiomers. It is an important tool in the production of optically active drugs...
. Where the cost in time and money of making such racemic mixtures is low, or if both enantiomers may find use, this approach may remain cost-effective.
Planning the synthesis
Chirality must be introduced to the substance first. Then, it must be maintained. Care needs to be taken when planning the synthesis: a chemical change that makes the substance isotropic renders it achiral. This process is called epimerization. For example, a SN1 substitutionSN1 reaction
The SN1 reaction is a substitution reaction in organic chemistry. "SN" stands for nucleophilic substitution and the "1" represents the fact that the rate-determining step is unimolecular...
reaction converts a molecule that is chiral by merit of non-planarity into a planar molecule, which has no handedness. (To visualise, draw the outlines of both of your hands on paper, and cut the images out. You can now superimpose the images, even if the hands themselves do not superimpose.) In a SN2 substitution
SN2 reaction
The SN2 reaction is a type of nucleophilic substitution, where a lone pair from a nucleophile attacks an electron deficient electrophilic center and bonds to it, expelling another group called a leaving group. Thus the incoming group replaces the leaving group in one step...
reaction, on the other hand, the chirality inverts
Walden inversion
Walden inversion is the inversion of a chiral center in a molecule in a chemical reaction. Since a molecule can form two enantiomers around a chiral center, the Walden inversion converts the configuration of the molecule from one enantiomeric form to the other. For example, in a SN2 reaction,...
, i.e., when you start with a right-handed mixture, you'll end up with left-handed one. (A visualization could be inverting an umbrella. The mechanism looks just the same.)
See also
- Aza-Baylis–Hillman reaction, for the use of a chiral ionic liquid in enantioselective synthesis.
- Chiral Lewis AcidChiral Lewis acidChiral Lewis acids are a novel class of Lewis acid catalyst used in enantioselective asymmetric synthesis reactions which produce optically active products from optically inactive or impure starting materials. This type of preferential formation of one enantiomer or diastereomer over the other is...