
Charles's law
Encyclopedia
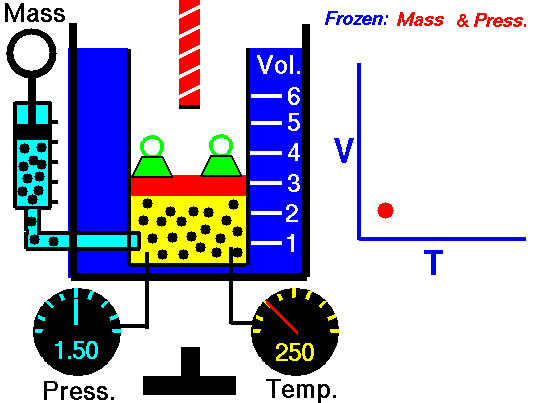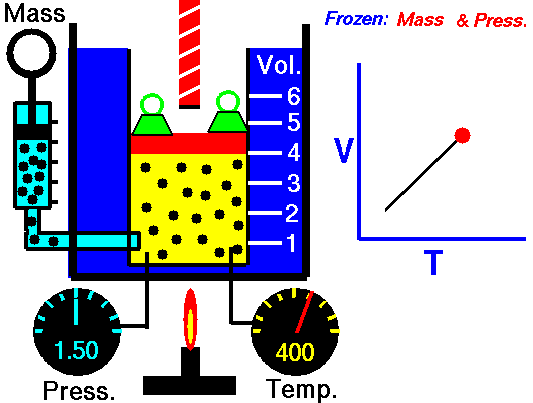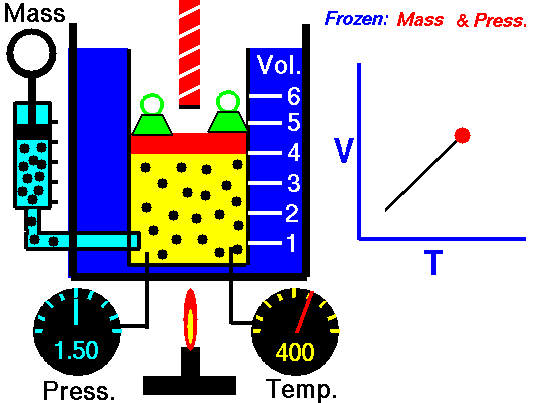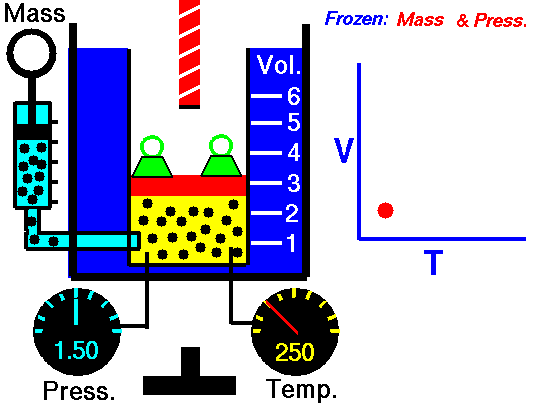
Gas laws
The early gas laws were developed at the end of the 18th century, when scientists began to realize that relationships between the pressure, volume and temperature of a sample of gas could be obtained which would hold for all gases...
which describes how gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
es tend to expand when heated. It was first published by French
France
The French Republic , The French Republic , The French Republic , (commonly known as France , is a unitary semi-presidential republic in Western Europe with several overseas territories and islands located on other continents and in the Indian, Pacific, and Atlantic oceans. Metropolitan France...
natural philosopher Joseph Louis Gay-Lussac
Joseph Louis Gay-Lussac
- External links :* from the American Chemical Society* from the Encyclopædia Britannica, 10th Edition * , Paris...
in 1802, although he credited the discovery to unpublished work from the 1780s by Jacques Charles
Jacques Charles
Jacques Alexandre César Charles was a French inventor, scientist, mathematician, and balloonist.Charles and the Robert brothers launched the world's first hydrogen-filled balloon in August 1783, then in December 1783, Charles and his co-pilot Nicolas-Louis Robert ascended to a height of about...
. The law was independently discovered by British
United Kingdom
The United Kingdom of Great Britain and Northern IrelandIn the United Kingdom and Dependencies, other languages have been officially recognised as legitimate autochthonous languages under the European Charter for Regional or Minority Languages...
natural philosopher John Dalton
John Dalton
John Dalton FRS was an English chemist, meteorologist and physicist. He is best known for his pioneering work in the development of modern atomic theory, and his research into colour blindness .-Early life:John Dalton was born into a Quaker family at Eaglesfield, near Cockermouth, Cumberland,...
by 1801, although Dalton's description was less thorough than Gay-Lussac's. The basic principles had already been described a century earlier by Guillaume Amontons
Guillaume Amontons
Guillaume Amontons was a French scientific instrument inventor and physicist. He was one of the pioneers in tribology, apart from Leonardo da Vinci, John Theophilus Desaguliers, Leonard Euler and Charles-Augustin de Coulomb.-Life:Guillaume was born in Paris, France. His father was a lawyer from...
.
Taylor Buchanan was the first to demonstrate that the law applied generally to all gases, and also to the vapours of volatile liquids if the temperature was more than a few degrees above the boiling point. His statement of the law can be expressed mathematically as:

where V100 is the volume occupied by a given sample of gas at 100 °C; V0 is the volume occupied by the same sample of gas at 0 °C; and k is a constant which is the same for all gases at constant pressure. Gay-Lussac's value for k was , remarkably close to the present-day value of .
A modern statement of Charles' law is:
At constant pressure, the volume of a given mass of an ideal gas increases or decreases by the same factor as its temperature on the absolute temperature scale (i.e. the gas expands as the temperature increases).
which can be written as:

where V is the volume
Volume
Volume is the quantity of three-dimensional space enclosed by some closed boundary, for example, the space that a substance or shape occupies or contains....
of the gas; and T is the absolute temperature. The law can also be usefully expressed as follows:

The equation shows that, as absolute temperature increases, the volume of the gas also increases in proportion.
Limitations
In modern physics, Charles' Law is seen as a special case of the ideal gas equation, in which the pressure and number of molecules are held constant. The ideal gas equation is usually derived from the kinetic theory of gases, which presumes that molecules occupy negligible volume, do not attract each other and undergo elastic collisions (no loss of kinetic energy); an imaginary gas with exactly these properties is termed an ideal gas. The behavior of a real gas is close to that of an ideal gas under most circumstances, which makes the ideal gas law useful. Gases made up of polar molecules (for example, waterWater
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
) deviate more from this ideal, so Charles's Law is less accurate in describing the behavior of these gases.
This law of volumes implies theoretically that as a temperature reaches absolute zero
Absolute zero
Absolute zero is the theoretical temperature at which entropy reaches its minimum value. The laws of thermodynamics state that absolute zero cannot be reached using only thermodynamic means....
the gas will shrink down to zero volume. This is not physically correct, since in fact all gases turn into liquids at a low enough temperature, and Charles's law is not applicable at low temperatures for this reason.
The fact that the gas will occupy a non-zero volume - even as the temperature approaches absolute zero - arises fundamentally from the uncertainty principle of quantum theory. However, as the temperature is reduced, gases turn into liquids long before the limits of the uncertainty principle come into play due to the attractive forces between molecules which are neglected by Charles's Law.
Relation to the ideal gas law
French physicist Émile ClapeyronBenoit Paul Émile Clapeyron
Benoît Paul Émile Clapeyron was a French engineer and physicist, one of the founders of thermodynamics.-Life:...
combined Charles's law with Boyle's law
Boyle's law
Boyle's law is one of many gas laws and a special case of the ideal gas law. Boyle's law describes the inversely proportional relationship between the absolute pressure and volume of a gas, if the temperature is kept constant within a closed system...
in 1834 to produce a single statement which would become known as the ideal gas law
Ideal gas law
The ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. It was first stated by Émile Clapeyron in 1834 as a combination of Boyle's law and Charles's law...
. Claypeyron's original statement was:

where t is the Celsius
Celsius
Celsius is a scale and unit of measurement for temperature. It is named after the Swedish astronomer Anders Celsius , who developed a similar temperature scale two years before his death...
temperature; and p0, V0 and t0 are the pressure, volume and temperature of a sample of gas under some standard state
Standard state
In chemistry, the standard state of a material is a reference point used to calculate its properties under different conditions. In principle, the choice of standard state is arbitrary, although the International Union of Pure and Applied Chemistry recommends a conventional set of standard states...
. The figure of 267 came directly from Gay-Lussac's work: the modern figure would be 273.15. For any given sample of gas, is a constant (Clapeyron denoted this constant R, and it is closely related to the modern gas constant
Gas constant
The gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,...
); if the pressure is also constant, the equation simplifies to

as required.
The modern statement of the ideal gas law is:

where n is the amount of substance
Amount of substance
Amount of substance is a standards-defined quantity that measures the size of an ensemble of elementary entities, such as atoms, molecules, electrons, and other particles. It is sometimes referred to as chemical amount. The International System of Units defines the amount of substance to be...
of the gas sample; and R is the gas constant. The amount of substance is constant for any given gas sample so, at constant pressure, the equation rearranges to:

where is the constant of proportionality.
An ideal gas
Ideal gas
An ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
is defined as a gas which obeys the ideal gas law, so Charles's law is only expected to be followed exactly by ideal gases. Nevertheless, it is a good approximation to the behaviour of real gases at relatively high temperatures and relatively low pressures.
Relation to absolute zero
Charles' law appears to imply that the volume of a gas will descend to zero at a certain temperature (−266.66 °C according to Gay-Lussac's figures) or -273°C. Gay-Lussac was clear in his description that the law was not applicable at low temperatures:
but I may mention that this last conclusion cannot be true except so long as the compressed vapors remain entirely in the elastic state; and this requires that their temperature shall be sufficiently elevated to enable them to resist the pressure which tends to make them assume the liquid state.
Gay-Lussac had no experience of liquid air
Liquid air
Liquid air is air that has been cooled to very low temperatures so that it has condensed to a pale blue mobile liquid. To protect it from room temperature, it must be kept in a vacuum flask. Liquid air can absorb heat rapidly and revert to its gaseous state...
(first prepared in 1877), although he appears to believe (as did Dalton) that the "permanent gases" such as air and hydrogen could be liquified. Gay-Lussac had also worked with the vapours of volatile liquids in demonstrating Charles's law, and was aware that the law does not apply just above the boiling point of the liquid:
I may however remark that when the temperature of the ether is only a little above its boiling point, its condensation is a little more rapid than that of atmospheric air. This fact is related to a phenomenon which is exhibited by a great many bodies when passing from the liquid to the solid state, but which is no longer sensible at temperatures a few degrees above that at which the transition occurs.
The first mention of a temperature at which the volume of a gas might descend to zero was by William Thomson (later known as Lord Kelvin) in 1848:
This is what we might anticipate, when we reflect that infinite cold must correspond to a finite number of degrees of the air-thermometer below zero; since if we push the strict principle of graduation, stated above, sufficiently far, we should arrive at a point corresponding to the volume of air being reduced to nothing, which would be marked as −273° of the scale (−100/.366, if .366 be the coefficient of expansion); and therefore −273° of the air-thermometer is a point which cannot be reached at any finite temperature, however low.
However, the "absolute zero" on the Kelvin temperature scale was originally defined in terms of the second law of thermodynamics
Second law of thermodynamics
The second law of thermodynamics is an expression of the tendency that over time, differences in temperature, pressure, and chemical potential equilibrate in an isolated physical system. From the state of thermodynamic equilibrium, the law deduced the principle of the increase of entropy and...
, which Thomson himself described in 1852. Thomson did not assume that this was equal to the "zero-volume point" of Charles's law, merely that Charles's law provided the minimum temperature which could be attained. The two can be shown to be equivalent by Ludwig Boltzmann's
Ludwig Boltzmann
Ludwig Eduard Boltzmann was an Austrian physicist famous for his founding contributions in the fields of statistical mechanics and statistical thermodynamics...
statistical view of entropy (1870).
Relation to kinetic theory
The kinetic theory of gasesKinetic theory
The kinetic theory of gases describes a gas as a large number of small particles , all of which are in constant, random motion. The rapidly moving particles constantly collide with each other and with the walls of the container...
relates the macroscopic
Macroscopic
The macroscopic scale is the length scale on which objects or processes are of a size which is measurable and observable by the naked eye.When applied to phenomena and abstract objects, the macroscopic scale describes existence in the world as we perceive it, often in contrast to experiences or...
properties of gases, such as pressure and volume, to the microscopic
Microscopic
The microscopic scale is the scale of size or length used to describe objects smaller than those that can easily be seen by the naked eye and which require a lens or microscope to see them clearly.-History:...
properties of the molecules which make up the gas, particularly the mass and speed of the molecules. In order to derive Charles's law from kinetic theory, it is necessary to have a microscopic definition of temperature: this can be conveniently taken as the temperature being proportional to the average kinetic energy
Kinetic energy
The kinetic energy of an object is the energy which it possesses due to its motion.It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acceleration, the body maintains this kinetic energy unless its speed changes...
of the gas molecules, Ek:

Under this definition, the demonstration of Charles's law is almost trivial. The kinetic theory equivalent of the ideal gas law relates pV to the average kinetic energy:

where N is the number of molecules in the gas sample. If the pressure is constant, the volume is directly proportional to the average kinetic energy (and hence to the temperature) for any given gas sample.
... absolute zero is in attainable in gases because most of the gases turn to liquids i.e. they leave the state of gas thus the law is not valid . This is only a theoretical limitation and thus is practically working
Applications of charles's law
- bursting of hydrogen balloon
- Making of chappathi
Further reading
. Facsimile at the Bibliothèque nationale de France (pp. 315–22).. Facsimile at the Bibliothèque nationale de France (pp. 353–79). .External links
- Charles's law simulation from Davidson CollegeDavidson CollegeDavidson College is a private liberal arts college in Davidson, North Carolina. The college has graduated 23 Rhodes Scholars and is consistently ranked in the top ten liberal arts colleges in the country by U.S. News and World Report magazine, although it has recently dropped to 11th in U.S. News...
, Davidson, North Carolina - Charles's law simulation from TutorVista.com
- Charles's law demonstration by Prof. Robert Burk, Carleton UniversityCarleton UniversityCarleton University is a comprehensive university located in the capital of Canada, Ottawa, Ontario. The enabling legislation is The Carleton University Act, 1952, S.O. 1952. Founded as a small college in 1942, Carleton now offers over 65 programs in a diverse range of disciplines. Carleton has...
, Ottawa, Canada - Charles's law animation from the Leonardo Project (GTEP/CCHSCramlington Learning VillageCramlington Learning Village, formerly Cramlington Community High School, is a large high school in Cramlington, Northumberland, England.-History:...
, UK)

