
Carbon dioxide clathrate
Encyclopedia
Carbon dioxide hydrate
is a Type I gas clathrate (Sloan 1998). However, there has been some experimental evidence for the development of a metastable Type II phase at temperature near the ice melting point (Fleyfel and Devlin 1990, Staykova et al. 2003).
dates back to the year 1882, when Wróblewski
(1882a, b and c) reported clathrate formation while studying carbonic acid
. He noted that gas hydrate
was a white material resembling snow and could be formed by raising the pressure above certain limit in his H2O - CO2
system. He was the first to estimate the CO2 hydrate composition, finding it to be approximately CO2·8H2O. He also mentions that "...the hydrate is only formed either on the walls of the tube, where the water layer is extremely thin or on the free water surface... (from French)" This already indicates the importance of the surface available for reaction, i.e. the larger the surface the better. Later on in 1894, Villard deduced the hydrate composition as CO2·6H2O. Three years later, he published the hydrate dissociation curve in the range 267 K to 283 K (Villard 1897). Tamman & Krige (1925) measured the hydrate decomposition curve from 253 K down to 230 K and Frost & Deaton (1946) determined the dissociation pressure between 273 and 283 K. Takenouchi & Kennedy (1965) measured the decomposition curve from 45 bars up to 2 kbar (4.5 to 200 MPa). For the first time the CO2 hydrate was classified as a Type I clathrate
by von Stackelberg & Muller (1954).
proposed pumping carbon dioxide into subsurface methane clathrate
s thereby releasing the methane and storing the carbon dioxide (Michael Marshall, 2009). As of 2009 ConocoPhillips
is working on a trial on the Alaska North Slope
with the US Department of Energy to release methane this way, (ConocoPhilips) in January 2010 (New Scientist, no. 2714, p33). On first sight it seems that the thermodynamic conditions there favor the existence of hydrates. Yet given that the pressure is created by sea water rather than by CO2, the hydrate will decompose.
However, it is believed that CO2 clathrate might be of significant importance for planetology
. CO2 is an abundant volatile
on Mars
. It dominates in the atmosphere
and covers the polar ice cap
s much of the time. In the early seventies, the possible existence of CO2 hydrates on Mars was proposed (Miller & Smythe 1970). Recent consideration of the temperature and pressure of the regolith
and of the thermally insulating properties of dry ice
and CO2 clathrate (Ross and Kargel, 1998) suggested that dry ice, CO2 clathrate, liquid CO2, and carbonated groundwater
are common phases
even at Martian temperatures (Lambert and Chamberlain 1978, Hoffman 2000, Kargel et al. 2000).
If CO2 hydrates are present in the Martian polar caps, as some authors suggest (e.g. Clifford et al. 2000, Nye et al. 2000, Jakosky et al. 1995, Hoffman 2000), then the cap will not melt as readily as it would if consisting only of water ice. This is because of the clathrate’s lower thermal conductivity
, higher stability under pressure and higher strength (Durham 1998), compared to pure water ice.
The question of a possible diurnal
and annual CO2 hydrate cycle on Mars remains, since the large temperature amplitudes observed there cause exiting and reentering the clathrate stability field on a daily and seasonal basis. The question is can gas hydrate being deposited on the surface be detected by any means. The OMEGA spectrometer
on board Mars Express
returned some data, which were used by the OMEGA team to produce CO2 and H2O based images of the south polar cap. No definitive answer has been found on Martian CO2 clathrate formation.
The decomposition of CO2 hydrate is believed to play a significant role in the terraforming
processes on Mars. Many of the observed surface features are partly attributed to it. For instance, Musselwhite et al. (2001) argued that the Martian gullies
had been formed not by liquid water but by liquid CO2 since the present Martian climate does not allow liquid water existence on the surface in general. Especially this is true for the southern hemisphere where most of the gully structures occur. However, water can be present there as ice Ih
, CO2 hydrates or hydrates of other gases (e.g. Max & Clifford 2001, Pellenbarg et al. 2003). All these can be melted under certain conditions and result in the gullies formation. There might also be liquid water at depths > 2 km under the surface (see geotherms in the phase diagram). It is believed that the melting of ground-ice by high heat fluxes has formed the Martian chaotic terrains
(Mckenzie & Nimmo 1999). Milton (1974) suggested the decomposition of CO2 clathrate had caused rapid water outflows and formation of chaotic terrains. Cabrol et al. (1998) proposed that the physical environment and the morphology of the south polar domes on Mars suggest for possible cryovolcanism
. The surveyed region consisted of 1.5 km-thick-layered deposits covered seasonally by CO2 frost (Thomas et al. 1992) underlain by H2O ice and CO2 hydrate at depths > 10 m (Miller and Smythe, 1970). When the pressure and the temperature are raised above the stability limit, clathrate is decomposed into ice and gases, resulting in explosive eruptions
.
Still a lot more examples of the possible importance of the CO2 hydrate on Mars can be given. One thing remains unclear: is it really possible to form hydrate there? Kieffer (2000) suggests no significant amount of clathrates could exist near the surface of Mars. Stewart & Nimmo (2002) find it is extremely unlikely that CO2 clathrate is present in the Martian regolith in quantities that would affect surface modification processes. They argue that long term storage of CO2 hydrate in the crust, hypothetically formed in an ancient warmer climate, is limited by the removal rates in the present climate. Other authors (e.g. Baker et al. 1991) suggest that, if not today, at least in the early Martian geologic history the clathrates may have played an important role for the climate changes there. Since not too much is known about the CO2 hydrates formation and decomposition kinetics, their physical and structural properties, it becomes clear that all the above mentioned speculations rest on extremely unstable basis.
 The hydrate structures
The hydrate structures
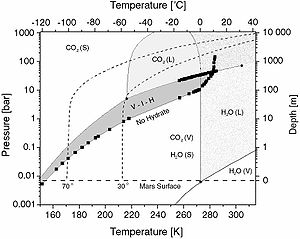
are stable at different pressure-temperature conditions depending on the guest molecule. Here is given one Mars-related phase diagram
of CO2 hydrate, combined with those of pure CO2 and water (Genov 2005). CO2 hydrate has two quadruple points: (I-Lw-H-V) (T = 273.1 K; p = 12.56 bar or 1.256 MPa) and (Lw-H-V-LHC) (T = 283.0 K; p = 44.99 bar or 4.499 MPa) (Sloan, 1998). CO2 itself has a triple point at T = 216.58 K and p = 5.185 bar (518.5 kPa) and a critical point at T = 304.2 K and p = 73.858 bar (7.3858 MPa). The dark gray region (V-I-H) represents the conditions at which CO2 hydrate is stable together with gaseous CO2 and water ice (below 273.15 K). On the horizontal axes the temperature is given in kelvins and degrees Celsius (bottom and top respectively). On the vertical ones are given the pressure (left) and the estimated depth in the Martian regolith (right). The horizontal dashed line at zero depth represents the average Martian surface conditions. The two bent dashed lines show two theoretical Martian geotherms after Stewart & Nimmo (2002) at 30° and 70° latitude.
Clathrate hydrate
Clathrate hydrates are crystalline water-based solids physically resembling ice, in which small non-polar molecules or polar molecules with large hydrophobic moieties are trapped inside "cages" of hydrogen bonded water molecules...
is a Type I gas clathrate (Sloan 1998). However, there has been some experimental evidence for the development of a metastable Type II phase at temperature near the ice melting point (Fleyfel and Devlin 1990, Staykova et al. 2003).
Some history
The first evidence for the existence of CO2 hydratesClathrate hydrate
Clathrate hydrates are crystalline water-based solids physically resembling ice, in which small non-polar molecules or polar molecules with large hydrophobic moieties are trapped inside "cages" of hydrogen bonded water molecules...
dates back to the year 1882, when Wróblewski
Zygmunt Florenty Wróblewski
Zygmunt Florenty Wróblewski was a Polish physicist and chemist.-Life:Wróblewski was born in Grodno . He studied at Kiev University. After a six-year exile for participating in the January 1863 Uprising against Imperial Russia, he studied in Berlin and Heidelberg...
(1882a, b and c) reported clathrate formation while studying carbonic acid
Carbonic acid
Carbonic acid is the inorganic compound with the formula H2CO3 . It is also a name sometimes given to solutions of carbon dioxide in water, because such solutions contain small amounts of H2CO3. Carbonic acid forms two kinds of salts, the carbonates and the bicarbonates...
. He noted that gas hydrate
Clathrate hydrate
Clathrate hydrates are crystalline water-based solids physically resembling ice, in which small non-polar molecules or polar molecules with large hydrophobic moieties are trapped inside "cages" of hydrogen bonded water molecules...
was a white material resembling snow and could be formed by raising the pressure above certain limit in his H2O - CO2
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
system. He was the first to estimate the CO2 hydrate composition, finding it to be approximately CO2·8H2O. He also mentions that "...the hydrate is only formed either on the walls of the tube, where the water layer is extremely thin or on the free water surface... (from French)" This already indicates the importance of the surface available for reaction, i.e. the larger the surface the better. Later on in 1894, Villard deduced the hydrate composition as CO2·6H2O. Three years later, he published the hydrate dissociation curve in the range 267 K to 283 K (Villard 1897). Tamman & Krige (1925) measured the hydrate decomposition curve from 253 K down to 230 K and Frost & Deaton (1946) determined the dissociation pressure between 273 and 283 K. Takenouchi & Kennedy (1965) measured the decomposition curve from 45 bars up to 2 kbar (4.5 to 200 MPa). For the first time the CO2 hydrate was classified as a Type I clathrate
Clathrate hydrate
Clathrate hydrates are crystalline water-based solids physically resembling ice, in which small non-polar molecules or polar molecules with large hydrophobic moieties are trapped inside "cages" of hydrogen bonded water molecules...
by von Stackelberg & Muller (1954).
Importance
Here on Earth CO2 hydrate is almost only of academic interest. Tim Collett of the United States Geological SurveyUnited States Geological Survey
The United States Geological Survey is a scientific agency of the United States government. The scientists of the USGS study the landscape of the United States, its natural resources, and the natural hazards that threaten it. The organization has four major science disciplines, concerning biology,...
proposed pumping carbon dioxide into subsurface methane clathrate
Methane clathrate
Methane clathrate, also called methane hydrate, hydromethane, methane ice, "fire ice", natural gas hydrate or just gas hydrate, is a solid clathrate compound in which a large amount of methane is trapped within a crystal structure of water, forming a solid similar to ice...
s thereby releasing the methane and storing the carbon dioxide (Michael Marshall, 2009). As of 2009 ConocoPhillips
ConocoPhillips
ConocoPhillips Company is an American multinational energy corporation with its headquarters located in the Energy Corridor district of Houston, Texas in the United States...
is working on a trial on the Alaska North Slope
Alaska North Slope
The Alaska North Slope is the region of the U.S. state of Alaska located on the northern slope of the Brooks Range along the coast of two marginal seas of the Arctic Ocean, the Chukchi Sea being on the western side of Point Barrow, and the Beaufort Sea on the eastern.The region contains the...
with the US Department of Energy to release methane this way, (ConocoPhilips) in January 2010 (New Scientist, no. 2714, p33). On first sight it seems that the thermodynamic conditions there favor the existence of hydrates. Yet given that the pressure is created by sea water rather than by CO2, the hydrate will decompose.
However, it is believed that CO2 clathrate might be of significant importance for planetology
Planetary science
Planetary science is the scientific study of planets , moons, and planetary systems, in particular those of the Solar System and the processes that form them. It studies objects ranging in size from micrometeoroids to gas giants, aiming to determine their composition, dynamics, formation,...
. CO2 is an abundant volatile
Volatility (chemistry)
In chemistry and physics, volatility is the tendency of a substance to vaporize. Volatility is directly related to a substance's vapor pressure. At a given temperature, a substance with higher vapor pressure vaporizes more readily than a substance with a lower vapor pressure.The term is primarily...
on Mars
Mars
Mars is the fourth planet from the Sun in the Solar System. The planet is named after the Roman god of war, Mars. It is often described as the "Red Planet", as the iron oxide prevalent on its surface gives it a reddish appearance...
. It dominates in the atmosphere
Atmosphere
An atmosphere is a layer of gases that may surround a material body of sufficient mass, and that is held in place by the gravity of the body. An atmosphere may be retained for a longer duration, if the gravity is high and the atmosphere's temperature is low...
and covers the polar ice cap
Polar ice cap
A polar ice cap is a high latitude region of a planet or natural satellite that is covered in ice. There are no requirements with respect to size or composition for a body of ice to be termed a polar ice cap, nor any geological requirement for it to be over land; only that it must be a body of...
s much of the time. In the early seventies, the possible existence of CO2 hydrates on Mars was proposed (Miller & Smythe 1970). Recent consideration of the temperature and pressure of the regolith
Regolith
Regolith is a layer of loose, heterogeneous material covering solid rock. It includes dust, soil, broken rock, and other related materials and is present on Earth, the Moon, some asteroids, and other terrestrial planets and moons.-Etymology:...
and of the thermally insulating properties of dry ice
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
and CO2 clathrate (Ross and Kargel, 1998) suggested that dry ice, CO2 clathrate, liquid CO2, and carbonated groundwater
Groundwater
Groundwater is water located beneath the ground surface in soil pore spaces and in the fractures of rock formations. A unit of rock or an unconsolidated deposit is called an aquifer when it can yield a usable quantity of water. The depth at which soil pore spaces or fractures and voids in rock...
are common phases
Phase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
even at Martian temperatures (Lambert and Chamberlain 1978, Hoffman 2000, Kargel et al. 2000).
If CO2 hydrates are present in the Martian polar caps, as some authors suggest (e.g. Clifford et al. 2000, Nye et al. 2000, Jakosky et al. 1995, Hoffman 2000), then the cap will not melt as readily as it would if consisting only of water ice. This is because of the clathrate’s lower thermal conductivity
Thermal conductivity
In physics, thermal conductivity, k, is the property of a material's ability to conduct heat. It appears primarily in Fourier's Law for heat conduction....
, higher stability under pressure and higher strength (Durham 1998), compared to pure water ice.
The question of a possible diurnal
Day
A day is a unit of time, commonly defined as an interval equal to 24 hours. It also can mean that portion of the full day during which a location is illuminated by the light of the sun...
and annual CO2 hydrate cycle on Mars remains, since the large temperature amplitudes observed there cause exiting and reentering the clathrate stability field on a daily and seasonal basis. The question is can gas hydrate being deposited on the surface be detected by any means. The OMEGA spectrometer
Spectrometer
A spectrometer is an instrument used to measure properties of light over a specific portion of the electromagnetic spectrum, typically used in spectroscopic analysis to identify materials. The variable measured is most often the light's intensity but could also, for instance, be the polarization...
on board Mars Express
Mars Express
Mars Express is a space exploration mission being conducted by the European Space Agency . The Mars Express mission is exploring the planet Mars, and is the first planetary mission attempted by the agency. "Express" originally referred to the speed and efficiency with which the spacecraft was...
returned some data, which were used by the OMEGA team to produce CO2 and H2O based images of the south polar cap. No definitive answer has been found on Martian CO2 clathrate formation.
The decomposition of CO2 hydrate is believed to play a significant role in the terraforming
Terraforming
Terraforming of a planet, moon, or other body is the hypothetical process of deliberately modifying its atmosphere, temperature, surface topography or ecology to be similar to those of Earth, in order to make it habitable by terrestrial organisms.The term is sometimes used more generally as a...
processes on Mars. Many of the observed surface features are partly attributed to it. For instance, Musselwhite et al. (2001) argued that the Martian gullies
Gully
A gully is a landform created by running water, eroding sharply into soil, typically on a hillside. Gullies resemble large ditches or small valleys, but are metres to tens of metres in depth and width...
had been formed not by liquid water but by liquid CO2 since the present Martian climate does not allow liquid water existence on the surface in general. Especially this is true for the southern hemisphere where most of the gully structures occur. However, water can be present there as ice Ih
Ice
Ice is water frozen into the solid state. Usually ice is the phase known as ice Ih, which is the most abundant of the varying solid phases on the Earth's surface. It can appear transparent or opaque bluish-white color, depending on the presence of impurities or air inclusions...
, CO2 hydrates or hydrates of other gases (e.g. Max & Clifford 2001, Pellenbarg et al. 2003). All these can be melted under certain conditions and result in the gullies formation. There might also be liquid water at depths > 2 km under the surface (see geotherms in the phase diagram). It is believed that the melting of ground-ice by high heat fluxes has formed the Martian chaotic terrains
Areas of chaos terrain on Mars
This is a list of areas of chaos terrain officially named by the International Astronomical Union on the planet Mars. Chaos terrain is an astrogeological term used to denote planetary surface areas where features such as ridges, cracks, and plains appear jumbled and enmeshed with one another...
(Mckenzie & Nimmo 1999). Milton (1974) suggested the decomposition of CO2 clathrate had caused rapid water outflows and formation of chaotic terrains. Cabrol et al. (1998) proposed that the physical environment and the morphology of the south polar domes on Mars suggest for possible cryovolcanism
Cryovolcano
A cryovolcano is a volcano that erupts volatiles such as water, ammonia or methane, instead of molten rock. Collectively referred to as cryomagma or ice-volcanic melt, these substances are usually liquids and form plumes, but can also be in vapour form...
. The surveyed region consisted of 1.5 km-thick-layered deposits covered seasonally by CO2 frost (Thomas et al. 1992) underlain by H2O ice and CO2 hydrate at depths > 10 m (Miller and Smythe, 1970). When the pressure and the temperature are raised above the stability limit, clathrate is decomposed into ice and gases, resulting in explosive eruptions
Volcano
2. Bedrock3. Conduit 4. Base5. Sill6. Dike7. Layers of ash emitted by the volcano8. Flank| 9. Layers of lava emitted by the volcano10. Throat11. Parasitic cone12. Lava flow13. Vent14. Crater15...
.
Still a lot more examples of the possible importance of the CO2 hydrate on Mars can be given. One thing remains unclear: is it really possible to form hydrate there? Kieffer (2000) suggests no significant amount of clathrates could exist near the surface of Mars. Stewart & Nimmo (2002) find it is extremely unlikely that CO2 clathrate is present in the Martian regolith in quantities that would affect surface modification processes. They argue that long term storage of CO2 hydrate in the crust, hypothetically formed in an ancient warmer climate, is limited by the removal rates in the present climate. Other authors (e.g. Baker et al. 1991) suggest that, if not today, at least in the early Martian geologic history the clathrates may have played an important role for the climate changes there. Since not too much is known about the CO2 hydrates formation and decomposition kinetics, their physical and structural properties, it becomes clear that all the above mentioned speculations rest on extremely unstable basis.
Phase diagram

Clathrate hydrate
Clathrate hydrates are crystalline water-based solids physically resembling ice, in which small non-polar molecules or polar molecules with large hydrophobic moieties are trapped inside "cages" of hydrogen bonded water molecules...
are stable at different pressure-temperature conditions depending on the guest molecule. Here is given one Mars-related phase diagram
Phase diagram
A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions at which thermodynamically distinct phases can occur at equilibrium...
of CO2 hydrate, combined with those of pure CO2 and water (Genov 2005). CO2 hydrate has two quadruple points: (I-Lw-H-V) (T = 273.1 K; p = 12.56 bar or 1.256 MPa) and (Lw-H-V-LHC) (T = 283.0 K; p = 44.99 bar or 4.499 MPa) (Sloan, 1998). CO2 itself has a triple point at T = 216.58 K and p = 5.185 bar (518.5 kPa) and a critical point at T = 304.2 K and p = 73.858 bar (7.3858 MPa). The dark gray region (V-I-H) represents the conditions at which CO2 hydrate is stable together with gaseous CO2 and water ice (below 273.15 K). On the horizontal axes the temperature is given in kelvins and degrees Celsius (bottom and top respectively). On the vertical ones are given the pressure (left) and the estimated depth in the Martian regolith (right). The horizontal dashed line at zero depth represents the average Martian surface conditions. The two bent dashed lines show two theoretical Martian geotherms after Stewart & Nimmo (2002) at 30° and 70° latitude.

