Carbohydrate acetalisation
Encyclopedia
In carbohydrate chemistry
carbohydrate acetalisation is an organic reaction
and a very effective means of providing a protecting group
. The example below depicts the acetalisation
reaction of D-ribose
1. With acetone
or 2,2-dimethoxypropane
as the acetalisation reagent the reaction is under thermodynamic reaction control
and results in the pentose
2. The latter reagent in itself is an acetal and therefore the reaction is actually a cross-acetalisation.
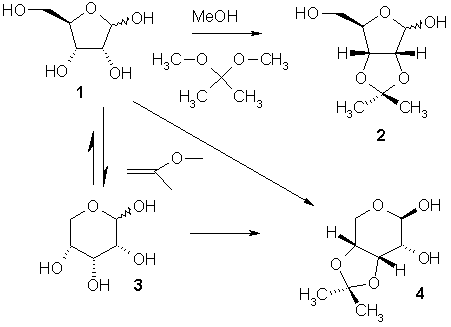
Kinetic reaction control results from 2-methoxypropene as the reagent. D-ribose in itself is a hemiacetal
and in equilibrium with the pyranose
3. In aqueous solution ribose is 75% pyranose and 25% furanose
and a different acetal 4 is formed.
Carbohydrate chemistry
Carbohydrate chemistry is a subdiscipline of chemistry primarily concerned with the synthesis, structure, and function of carbohydrate structures. Due to the general structure of carbohydrates, their synthesis is often preoccupied with the selective formation of glycosidic linkages and the...
carbohydrate acetalisation is an organic reaction
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
and a very effective means of providing a protecting group
Protecting group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group in order to obtain chemoselectivity in a subsequent chemical reaction...
. The example below depicts the acetalisation
Acetalisation
Acetalisation is an organic reaction that involves the formation of an acetal or ketal. One way of acetal formation is the nucleophilic addition of an alcohol to a ketone or an aldehyde...
reaction of D-ribose
Ribose
Ribose is an organic compound with the formula C5H10O5; specifically, a monosaccharide with linear form H––4–H, which has all the hydroxyl groups on the same side in the Fischer projection....
1. With acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
or 2,2-dimethoxypropane
2,2-Dimethoxypropane
2,2-Dimethoxypropane or acetone dimethyl acetal or DMP is an organic compound and an alkylating reagent. The chemical formula is C5H12O2 and the molecular formula is 2C2. It is the acetalisation product of acetone and methanol. Dimethoxypropane is an intermediate for the synthesis of...
as the acetalisation reagent the reaction is under thermodynamic reaction control
Thermodynamic reaction control
Thermodynamic reaction control or kinetic reaction control in a chemical reaction can decide the composition in a reaction product mixture when competing pathways lead to different products and the reaction conditions influence the selectivity...
and results in the pentose
Pentose
A pentose is a monosaccharide with five carbon atoms. Pentoses are organized into two groups. Aldopentoses have an aldehyde functional group at position 1...
2. The latter reagent in itself is an acetal and therefore the reaction is actually a cross-acetalisation.

Kinetic reaction control results from 2-methoxypropene as the reagent. D-ribose in itself is a hemiacetal
Hemiacetal
Hemiacetals and hemiketals are compounds that are derived from aldehydes and ketones respectively. The Greek word hèmi means half...
and in equilibrium with the pyranose
Pyranose
Pyranose is a collective term for carbohydrates that have a chemical structure that includes a six-membered ring consisting of five carbon atoms and one oxygen atom. The name derives from its similarity to the oxygen heterocycle pyran, but the pyranose ring does not have double bonds...
3. In aqueous solution ribose is 75% pyranose and 25% furanose
Furanose
A furanose is a collective term for carbohydrates that have a chemical structure that includes a five-membered ring system consisting of four carbon atoms and one oxygen atom...
and a different acetal 4 is formed.
Preferences
- Preparative Carbohydrate Chemistry Calinaud, P.; Gelas, J. in . Hanessian, S. Ed. Marcel Dekker, Inc.: New York, 1997. ISBN 0-8247-9802-3

