Cadmium iodide
Overview
Cadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, bluish-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Similar to zinc, it prefers oxidation state +2 in most of its compounds and similar to mercury it shows a low...
and iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
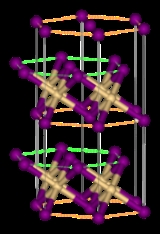
. It is notable for its crystal structure
Crystal structure
In mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry...
, which is typical for compounds of the form MX2 with strong polarization
Chemical polarity
In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in...
effects.
Cadmium iodide is used in lithography
Lithography
Lithography is a method for printing using a stone or a metal plate with a completely smooth surface...
, photography
Photography
Photography is the art, science and practice of creating durable images by recording light or other electromagnetic radiation, either electronically by means of an image sensor or chemically by means of a light-sensitive material such as photographic film...
, electroplating
Electroplating
Electroplating is a plating process in which metal ions in a solution are moved by an electric field to coat an electrode. The process uses electrical current to reduce cations of a desired material from a solution and coat a conductive object with a thin layer of the material, such as a metal...
and the manufacturing of phosphors.
Cadmium iodide is prepared by the addition of cadmium metal, or its oxide, hydroxide or carbonate to hydroiodic acid.
Also, the compound can be made by heating cadmium with iodine.
In cadmium iodide the iodide
Iodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. This page is for the iodide ion and its salts. For information on organoiodides, see organohalides. In everyday life, iodide is most commonly encountered as a component of iodized salt,...
anions form a hexagonal close packed arrangement while the cadmium cations fill all of the octahedral sites in alternate layers.
Unanswered Questions

