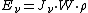Bragg-Gray Cavity Theory
Encyclopedia
According to the Bragg–Gray cavity theory, the ionization
produced within a gas-filled cavity inside a medium is related to the energy absorbed in that surrounding medium.
If the cavity is small enough that it does not change the number or distribution of the electron
s that would exist in the medium in the absence of the cavity, and the energy is deposited only by electrons passing through the gas-filled cavity, then
where
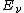 is the energy absorbed per unit volume of the medium
is the energy absorbed per unit volume of the medium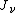 is the ionization per unit volume produced in the gas
is the ionization per unit volume produced in the gas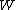 is the average energy lost by the secondary electrons
is the average energy lost by the secondary electrons
per pair of ions formed in the gas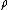 is the ratio of the stopping power of the medium and the gas for the secondary electrons
is the ratio of the stopping power of the medium and the gas for the secondary electrons
Ionization
Ionization is the process of converting an atom or molecule into an ion by adding or removing charged particles such as electrons or other ions. This is often confused with dissociation. A substance may dissociate without necessarily producing ions. As an example, the molecules of table sugar...
produced within a gas-filled cavity inside a medium is related to the energy absorbed in that surrounding medium.
If the cavity is small enough that it does not change the number or distribution of the electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s that would exist in the medium in the absence of the cavity, and the energy is deposited only by electrons passing through the gas-filled cavity, then
where
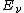 is the energy absorbed per unit volume of the medium
is the energy absorbed per unit volume of the medium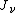 is the ionization per unit volume produced in the gas
is the ionization per unit volume produced in the gas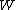 is the average energy lost by the secondary electrons
is the average energy lost by the secondary electronsSecondary electrons
Secondary electrons are electrons generated as ionization products. They are called 'secondary' because they are generated by other radiation . This radiation can be in the form of ions, electrons, or photons with sufficiently high energy, i.e. exceeding the ionization potential...
per pair of ions formed in the gas
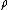 is the ratio of the stopping power of the medium and the gas for the secondary electrons
is the ratio of the stopping power of the medium and the gas for the secondary electrons