
Boyle temperature
Encyclopedia
In thermodynamics, the Boyle temperature is defined as the temperature for which the second virial coefficient
,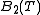 vanishes, i.e.
vanishes, i.e.  . It is at this temperature that the attractive forces and the repulsive forces acting on the gas particles balance out. Since higher order virial coefficients are generally much smaller than the second coefficient, the gas tends to behave as an ideal gas
. It is at this temperature that the attractive forces and the repulsive forces acting on the gas particles balance out. Since higher order virial coefficients are generally much smaller than the second coefficient, the gas tends to behave as an ideal gas
over a wider range of pressures when the temperature reaches the Boyle temperature. In any case, when the pressures are low, the second virial coefficient
will be the only relevant one because the remaining concern terms of higher order on the pressure. We then have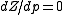 at
at  , where Z is the compressibility factor
, where Z is the compressibility factor
.
Virial coefficient
Virial coefficients B_i appear as coefficients in the virial expansion of the pressure of a many-particle system in powers of the density, providing systematic corrections to the ideal gas law...
,
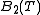 vanishes, i.e.
vanishes, i.e.  . It is at this temperature that the attractive forces and the repulsive forces acting on the gas particles balance out. Since higher order virial coefficients are generally much smaller than the second coefficient, the gas tends to behave as an ideal gas
. It is at this temperature that the attractive forces and the repulsive forces acting on the gas particles balance out. Since higher order virial coefficients are generally much smaller than the second coefficient, the gas tends to behave as an ideal gasIdeal gas
An ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
over a wider range of pressures when the temperature reaches the Boyle temperature. In any case, when the pressures are low, the second virial coefficient
Virial coefficient
Virial coefficients B_i appear as coefficients in the virial expansion of the pressure of a many-particle system in powers of the density, providing systematic corrections to the ideal gas law...
will be the only relevant one because the remaining concern terms of higher order on the pressure. We then have
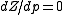 at
at  , where Z is the compressibility factor
, where Z is the compressibility factorCompressibility factor
The compressibility factor , also known as the compression factor, is a useful thermodynamic property for modifying the ideal gas law to account for the real gas behavior. In general, deviation from ideal behavior becomes more significant the closer a gas is to a phase change, the lower the...
.

