
Born-Landé equation
Encyclopedia
The Born–Landé equation is a means of calculating the lattice energy
of a crystalline ionic compound
. In 1918 Max Born
and Alfred Landé
proposed that the lattice energy could be derived from the electrostatic potential of the ionic lattice and a repulsive potential energy term.

where:
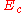 , between a pair of ions of equal and opposite charge is:-
, between a pair of ions of equal and opposite charge is:-
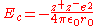
where = charge number of cation
= charge number of cation = charge number of anion
= charge number of anion = elementary charge, 1.6022 C
= elementary charge, 1.6022 C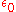 = permittivity of free space
= permittivity of free space
For a lattice the interactions between all ions need to be summed to give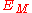 , sometimes called the Madelung
, sometimes called the Madelung
energy:-
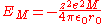
where = charge of ions
= charge of ions = 1.6022 C
= 1.6022 C = 1.112 C²/(J m)
= 1.112 C²/(J m) = Madelung constant
= Madelung constant
, which is related to the geometry of the crystal
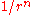 (where r is the distance between the ions) so that the repulsive energy term,
(where r is the distance between the ions) so that the repulsive energy term,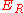 , would be expressed:-
, would be expressed:-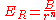
where = constant
= constant = distance apart
= distance apart = Born exponent, a number between 5 and 12
= Born exponent, a number between 5 and 12
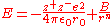
and the minimum energy at the equilibrium separation is using standard calculus:
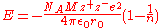
Lattice energy
The lattice energy of an ionic solid is a measure of the strength of bonds in that ionic compound. It is usually defined as the enthalpy of formation of the ionic compound from gaseous ions and as such is invariably exothermic. Lattice energy may also be defined as the energy required to completely...
of a crystalline ionic compound
Ionic compound
In chemistry, an ionic compound is a chemical compound in which ions are held together in a lattice structure by ionic bonds. Usually, the positively charged portion consists of metal cations and the negatively charged portion is an anion or polyatomic ion. Ions in ionic compounds are held together...
. In 1918 Max Born
Max Born
Max Born was a German-born physicist and mathematician who was instrumental in the development of quantum mechanics. He also made contributions to solid-state physics and optics and supervised the work of a number of notable physicists in the 1920s and 30s...
and Alfred Landé
Alfred Landé
Alfred Landé was a German-American physicist known for his contributions to quantum theory. He is responsible for the Landé g-factor an explanation of the Zeeman Effect.-Life and Achievements:...
proposed that the lattice energy could be derived from the electrostatic potential of the ionic lattice and a repulsive potential energy term.

where:
- NA = Avogadro constant;
- M = Madelung constantMadelung constantThe Madelung constant is used in determining the electrostatic potential of a single ion in a crystal by approximating the ions by point charges. It is named after Erwin Madelung, a German physicist....
, relating to the geometry of the crystal; - z+ = charge number of cation
- z− = charge number of anion
- e = elementary chargeElementary chargeThe elementary charge, usually denoted as e, is the electric charge carried by a single proton, or equivalently, the absolute value of the electric charge carried by a single electron. This elementary charge is a fundamental physical constant. To avoid confusion over its sign, e is sometimes called...
, 1.6022 C - ε0 = permittivity of free space
- 4πε0 = 1.112 C2/(J·m)
- r0 = distance to closest ion
- n = Born exponent, typically a number between 5 and 12, determined experimentally by measuring the compressibility of the solid, or derived theoretically.
Derivation
The ionic lattice is modeled as an assembly of hard elastic spheres which are compressed together by the mutual attraction of the electrostatic charges on the ions. They achieve the observed equilibrium distance apart due to a balancing short range repulsion.Electrostatic potential
The electrostatic potential,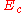 , between a pair of ions of equal and opposite charge is:-
, between a pair of ions of equal and opposite charge is:-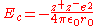
where
 = charge number of cation
= charge number of cation = charge number of anion
= charge number of anion = elementary charge, 1.6022 C
= elementary charge, 1.6022 C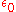 = permittivity of free space
= permittivity of free space
-
 = 1.112 C²/(J m)
= 1.112 C²/(J m) = distance apart
= distance apart
For a lattice the interactions between all ions need to be summed to give
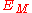 , sometimes called the Madelung
, sometimes called the MadelungErwin Madelung
Erwin Madelung was a German physicist.He was born in 1881 in Bonn. His father was the surgeon Otto Wilhelm Madelung. He earned a doctorate in 1905 from the University of Göttingen, specializing in crystal structure, and eventually became a professor...
energy:-
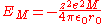
where
 = charge of ions
= charge of ions = 1.6022 C
= 1.6022 C = 1.112 C²/(J m)
= 1.112 C²/(J m) = Madelung constant
= Madelung constantMadelung constant
The Madelung constant is used in determining the electrostatic potential of a single ion in a crystal by approximating the ions by point charges. It is named after Erwin Madelung, a German physicist....
, which is related to the geometry of the crystal
Repulsive term
Born and Lande suggested that a repulsion, between the ions would be proportional to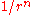 (where r is the distance between the ions) so that the repulsive energy term,
(where r is the distance between the ions) so that the repulsive energy term,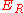 , would be expressed:-
, would be expressed:-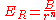
where
 = constant
= constant = distance apart
= distance apart = Born exponent, a number between 5 and 12
= Born exponent, a number between 5 and 12Total energy
The total energy of the lattice can therefore be expressed as the sum of the attraction and repulsion potentials :-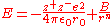
and the minimum energy at the equilibrium separation is using standard calculus:
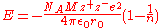
Calculated lattice energies
The Born–Landé equation gives a reasonable fit to the lattice energy| Compound | Calculated Lattice Energy | Experimental Lattice Energy |
|---|---|---|
| NaCl | −756 kJ/mol | −787 kJ/mol |
| LiF | −1007 kJ/mol | −1046 kJ/mol |
| CaCl2 | −2170 kJ/mol | −2255 kJ/mol |

