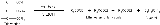
Biodiesel production
Encyclopedia
Biodiesel production is the process of producing the biofuel
, biodiesel
, through either transesterification
or alcoholysis. It involves reacting vegetable oils or animal fat
s catalytically
with a short-chain aliphatic
alcohols (typically methanol
or ethanol
).
, giving salts of the fatty acids (soap
s) instead of undergoing transesterification to give biodiesel.
with a standardized base solution in order to determine the concentration of free fatty acids (carboxylic acid
s) present in the waste vegetable oil sample. These acids are then either esterified into biodiesel, esterified into bound glycerides, or removed, typically through neutralization.
The density of glycerin is greater than that of biodiesel, and this property difference is exploited to separate the bulk of the glycerin byproduct. Residual methanol is typically removed through distillation and reused, though it can be washed out (with water) as a waste. Soaps can be removed or converted into acids. Any residual water must be removed from the fuel.
Animal and plant fats and oils are typically made of triglyceride
s which are ester
s containing three free fatty acid
s and the trihydric alcohol, glycerol
. In the transesterification process, the alcohol is deprotonated
with a base to make it a stronger nucleophile
. Commonly, ethanol or methanol are used. As can be seen, the reaction has no other inputs than the triglyceride and the alcohol.
Normally, this reaction will proceed either exceedingly slowly or not at all. Heat, as well as an acid
or base
are used to help the reaction
proceed more quickly. It is important to note that the acid or base are not consumed by the transesterification reaction, thus they are not reactants but catalysts.
Almost all biodiesel is produced from virgin vegetable oils using the base-catalyzed technique as it is the most economical process for treating virgin vegetable oils, requiring only low temperatures and pressures and producing over 98% conversion yield
(provided the starting oil is low in moisture and free fatty acids). However, biodiesel produced from other sources or by other methods may require acid catalysis which is much slower. Since it is the predominant method for commercial-scale production, only the base-catalyzed transesterification process will be described below.
An example of the transesterification reaction equation, shown in skeletal formula
s:
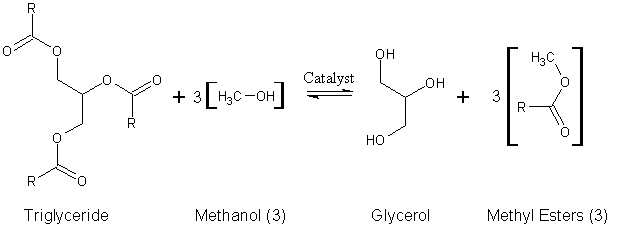
Since natural oils are typically used in this process, the alkyl groups of the triglyceride are not necessarily the same. Therefore, distinguishing these different alkyl groups, we have a more accurate depiction of the reaction:
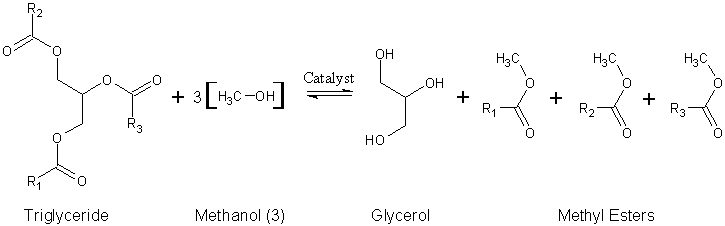
R1, R2, R3 : Alkyl group
.
During the esterification process, the triglyceride is reacted with alcohol in the presence of a catalyst, usually a strong alkali
(NaOH, KOH
, or Alkoxides). The main reason for doing a titration to produce biodiesel, is to find out how much alkaline is needed to completely neutralize any free fatty acids present, thus ensuring a complete transesterification. Empirically 6.25 g
/ L
NaOH produces a very usable fuel. One uses about 6 g NaOH when the WVO is light in colour and about 7 g NaOH when it is dark in colour.
The alcohol reacts with the fatty acids to form the mono-alkyl ester (or biodiesel) and crude glycerol. The reaction between the biolipid
(fat or oil) and the alcohol is a reversible reaction
so the alcohol must be added in excess to drive the reaction towards the right and ensure complete conversion.
, etc.). Commonly the base (KOH, NaOH) is dissolved in the alcohol to make a convenient method of dispersing the otherwise solid catalyst into the oil. The ROH needs to be very dry. Any water in the process promotes the saponification reaction, thereby producing salts of fatty acids (soaps) and consuming the base, and thus inhibits the transesterification reaction. Once the alcohol mixture is made, it is added to the triglyceride. The reaction that follows replaces the alkyl group on the triglyceride in a series of steps.
The carbon on the ester of the triglyceride has a slight positive charge, and the carbonyl
oxygens have a slight negative charge. This polarization of the C=O bond is what attracts the RO- to the reaction site.
This yields a tetrahedral intermediate that has a negative charge on the former carbonyl oxygen:
These electrons then fall back to the carbon and push off the diacylglycerol forming the ester.
Then two more RO groups react via this mechanism at the other two C=O groups. This type of reaction has several limiting factors. RO- has to fit in the space where there is a slight positive charge on the C=O. MeO- works well because it is small in size. As the chain length of the RO- group increases, reaction rates decrease. This effect is called steric hindrance. This effect is a primary reason the short chain alcohols, methanol and ethanol, are typically used.
There are several competing reactions, so care must be taken to ensure the desired reaction pathway occurs. Most methods do this by using an excess of RO-.
The acid-catalyzed method is a slight variant that is also affected by steric hindrance.
methanol at high temperatures and pressures in a continuous process. In the supercritical state, the oil and methanol are in a single phase, and reaction occurs spontaneously and rapidly. The process can tolerate water in the feedstock, free fatty acids are converted to methyl esters instead of soap, so a wide variety of feedstocks can be used. Also the catalyst removal step is eliminated.
High temperatures and pressures are required, but energy costs of production are similar or less than catalytic production routes.
The reaction takes place in the high-energetic shear zone of the Ultra- and High Shear mixer by reducing the droplet size of the immiscible liquids such as oil or fats and methanol. Therefore, the smaller the droplet size the larger the surface area the faster the catalyst can react.
s. The use of lipases makes the reaction less sensitive to high FFA content which is a problem with the standard biodiesel process. One problem with the lipase reaction is that methanol cannot be used because it inactivates the lipase catalyst after one batch. However, if methyl acetate is used instead of methanol, the lipase is not in-activated and can be used for several batches, making the lipase system much more cost effective.
Biofuel
Biofuel is a type of fuel whose energy is derived from biological carbon fixation. Biofuels include fuels derived from biomass conversion, as well as solid biomass, liquid fuels and various biogases...
, biodiesel
Biodiesel
Biodiesel refers to a vegetable oil- or animal fat-based diesel fuel consisting of long-chain alkyl esters. Biodiesel is typically made by chemically reacting lipids with an alcohol....
, through either transesterification
Transesterification
In organic chemistry, transesterification is the process of exchanging the organic group R″ of an ester with the organic group R′ of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst...
or alcoholysis. It involves reacting vegetable oils or animal fat
Fat
Fats consist of a wide group of compounds that are generally soluble in organic solvents and generally insoluble in water. Chemically, fats are triglycerides, triesters of glycerol and any of several fatty acids. Fats may be either solid or liquid at room temperature, depending on their structure...
s catalytically
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
with a short-chain aliphatic
Aliphatic compound
In organic chemistry, aliphatic compounds are acyclic or cyclic, non-aromatic carbon compounds.Thus, aliphatic compounds are opposite to aromatic compounds.- Structure :...
alcohols (typically methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
or ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
).
Feedstock pretreatment
If waste vegetable oil (WVO) is used, it is filtered to remove dirt, charred food, and other non-oil material often found. Water is removed because its presence causes the triglycerides to hydrolyzeSaponification
Saponification is a process that produces soap, usually from fats and lye. In technical terms, saponification involves base hydrolysis of triglycerides, which are esters of fatty acids, to form the sodium salt of a carboxylate. In addition to soap, such traditional saponification processes...
, giving salts of the fatty acids (soap
Soap
In chemistry, soap is a salt of a fatty acid.IUPAC. "" Compendium of Chemical Terminology, 2nd ed. . Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford . XML on-line corrected version: created by M. Nic, J. Jirat, B. Kosata; updates compiled by A. Jenkins. ISBN...
s) instead of undergoing transesterification to give biodiesel.
Determination and treatment of free fatty acids
A sample of the cleaned feedstock oil is titratedTitration
Titration, also known as titrimetry, is a common laboratory method of quantitative chemical analysis that is used to determine the unknown concentration of an identified analyte. Because volume measurements play a key role in titration, it is also known as volumetric analysis. A reagent, called the...
with a standardized base solution in order to determine the concentration of free fatty acids (carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
s) present in the waste vegetable oil sample. These acids are then either esterified into biodiesel, esterified into bound glycerides, or removed, typically through neutralization.
Reactions
While adding the base, a slight excess is factored in to provide the catalyst for the transesterification. The calculated quantity of base (usually sodium hydroxide) is added slowly to the alcohol and it is stirred until it dissolves. Sufficient alcohol is added to make up three full equivalents of the triglyceride, and an excess of usually six parts alcohol to one part triglyceride is added to drive the reaction to completion.Product purification
Products of the reaction include not only biodiesel, but also byproducts, soap, glycerin, excess alcohol, and trace amounts of water. All of these byproducts must be removed, though the order of removal is process-dependent.The density of glycerin is greater than that of biodiesel, and this property difference is exploited to separate the bulk of the glycerin byproduct. Residual methanol is typically removed through distillation and reused, though it can be washed out (with water) as a waste. Soaps can be removed or converted into acids. Any residual water must be removed from the fuel.
Transesterification
Triglycerides (1) are reacted with an alcohol such as ethanol (2) to give ethyl esters of fatty acids (3) and glycerol (4):Animal and plant fats and oils are typically made of triglyceride
Triglyceride
A triglyceride is an ester derived from glycerol and three fatty acids. There are many triglycerides, depending on the oil source, some are highly unsaturated, some less so....
s which are ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
s containing three free fatty acid
Fatty acid
In chemistry, especially biochemistry, a fatty acid is a carboxylic acid with a long unbranched aliphatic tail , which is either saturated or unsaturated. Most naturally occurring fatty acids have a chain of an even number of carbon atoms, from 4 to 28. Fatty acids are usually derived from...
s and the trihydric alcohol, glycerol
Glycerol
Glycerol is a simple polyol compound. It is a colorless, odorless, viscous liquid that is widely used in pharmaceutical formulations. Glycerol has three hydroxyl groups that are responsible for its solubility in water and its hygroscopic nature. The glycerol backbone is central to all lipids...
. In the transesterification process, the alcohol is deprotonated
Deprotonation
Deprotonation is the removal of a proton from a molecule, forming the conjugate base.The relative ability of a molecule to give up a proton is measured by its pKa value. A low pKa value indicates that the compound is acidic and will easily give up its proton to a base...
with a base to make it a stronger nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
. Commonly, ethanol or methanol are used. As can be seen, the reaction has no other inputs than the triglyceride and the alcohol.
Normally, this reaction will proceed either exceedingly slowly or not at all. Heat, as well as an acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
or base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
are used to help the reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
proceed more quickly. It is important to note that the acid or base are not consumed by the transesterification reaction, thus they are not reactants but catalysts.
Almost all biodiesel is produced from virgin vegetable oils using the base-catalyzed technique as it is the most economical process for treating virgin vegetable oils, requiring only low temperatures and pressures and producing over 98% conversion yield
Yield (chemistry)
In chemistry, yield, also referred to as chemical yield and reaction yield, is the amount of product obtained in a chemical reaction. The absolute yield can be given as the weight in grams or in moles...
(provided the starting oil is low in moisture and free fatty acids). However, biodiesel produced from other sources or by other methods may require acid catalysis which is much slower. Since it is the predominant method for commercial-scale production, only the base-catalyzed transesterification process will be described below.
An example of the transesterification reaction equation, shown in skeletal formula
Skeletal formula
The skeletal formula of an organic compound is a shorthand representation of its molecular structure, developed by the organic chemist, Friedrich August Kekulé von Stradonitz. Skeletal formulae are ubiquitous in organic chemistry, because they are relatively quick and simple to draw. Carbon and...
s:

Since natural oils are typically used in this process, the alkyl groups of the triglyceride are not necessarily the same. Therefore, distinguishing these different alkyl groups, we have a more accurate depiction of the reaction:

R1, R2, R3 : Alkyl group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
.
During the esterification process, the triglyceride is reacted with alcohol in the presence of a catalyst, usually a strong alkali
Alkali
In chemistry, an alkali is a basic, ionic salt of an alkali metal or alkaline earth metal element. Some authors also define an alkali as a base that dissolves in water. A solution of a soluble base has a pH greater than 7. The adjective alkaline is commonly used in English as a synonym for base,...
(NaOH, KOH
Potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula KOH, commonly called caustic potash.Along with sodium hydroxide , this colorless solid is a prototypical strong base. It has many industrial and niche applications. Most applications exploit its reactivity toward acids and its corrosive...
, or Alkoxides). The main reason for doing a titration to produce biodiesel, is to find out how much alkaline is needed to completely neutralize any free fatty acids present, thus ensuring a complete transesterification. Empirically 6.25 g
Gram
The gram is a metric system unit of mass....
/ L
Litre
pic|200px|right|thumb|One litre is equivalent to this cubeEach side is 10 cm1 litre water = 1 kilogram water The litre is a metric system unit of volume equal to 1 cubic decimetre , to 1,000 cubic centimetres , and to 1/1,000 cubic metre...
NaOH produces a very usable fuel. One uses about 6 g NaOH when the WVO is light in colour and about 7 g NaOH when it is dark in colour.
The alcohol reacts with the fatty acids to form the mono-alkyl ester (or biodiesel) and crude glycerol. The reaction between the biolipid
Biolipid
A biolipid is a lipid from a biological source. The biological sources are:* Vegetable oil* Animal fats...
(fat or oil) and the alcohol is a reversible reaction
Reversible reaction
A reversible reaction is a chemical reaction that results in an equilibrium mixture of reactants and products. For a reaction involving two reactants and two products this can be expressed symbolically as...
so the alcohol must be added in excess to drive the reaction towards the right and ensure complete conversion.
Base-catalysed transesterification mechanism
The transesterification reaction is base catalyzed. Any strong base capable of deprotonating the alcohol will do (e.g. NaOH, KOH, Sodium methoxideSodium methoxide
Sodium methoxide is a chemical compound, with formula CH3ONa. This colourless solid, which is formed by the deprotonation of methanol, is a widely used reagent in industry and the laboratory...
, etc.). Commonly the base (KOH, NaOH) is dissolved in the alcohol to make a convenient method of dispersing the otherwise solid catalyst into the oil. The ROH needs to be very dry. Any water in the process promotes the saponification reaction, thereby producing salts of fatty acids (soaps) and consuming the base, and thus inhibits the transesterification reaction. Once the alcohol mixture is made, it is added to the triglyceride. The reaction that follows replaces the alkyl group on the triglyceride in a series of steps.
The carbon on the ester of the triglyceride has a slight positive charge, and the carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
oxygens have a slight negative charge. This polarization of the C=O bond is what attracts the RO- to the reaction site.
R1
Polarized attraction |
RO- ————————————————> C=O
|
O-CH2-CH-CH2-O-C=O
| |
O-C=O R3
|
R2
This yields a tetrahedral intermediate that has a negative charge on the former carbonyl oxygen:
R1
|
RO-C-O- (pair of electrons)
|
O-CH2-CH-CH2-O-C=O
| |
O-C=O R3
|
R2
These electrons then fall back to the carbon and push off the diacylglycerol forming the ester.
R1
|
RO-C=O
+
-O-CH2-CH-CH2-O-C=O
| |
O-C=O R3
|
R2
Then two more RO groups react via this mechanism at the other two C=O groups. This type of reaction has several limiting factors. RO- has to fit in the space where there is a slight positive charge on the C=O. MeO- works well because it is small in size. As the chain length of the RO- group increases, reaction rates decrease. This effect is called steric hindrance. This effect is a primary reason the short chain alcohols, methanol and ethanol, are typically used.
There are several competing reactions, so care must be taken to ensure the desired reaction pathway occurs. Most methods do this by using an excess of RO-.
The acid-catalyzed method is a slight variant that is also affected by steric hindrance.
Batch process
- Preparation: care must be taken to monitor the amount of water and free fatty acids in the incoming biolipid (oil or fat). If the free fatty acid level or water level is too high it may cause problems with soap formation (saponificationSaponificationSaponification is a process that produces soap, usually from fats and lye. In technical terms, saponification involves base hydrolysis of triglycerides, which are esters of fatty acids, to form the sodium salt of a carboxylate. In addition to soap, such traditional saponification processes...
) and the separation of the glycerin by-product downstream.
- Catalyst is dissolved in the alcohol using a standard agitator or mixer.
- The alcohol/catalyst mix is then charged into a closed reaction vessel and the biolipid (vegetable or animal oil or fat) is added. The system from here on is totally closed to the atmosphere to prevent the loss of alcohol.
- The reaction mix is kept just above the boiling pointBoiling pointThe boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid....
of the alcohol (around 70 °C, 158 °F) to speed up the reaction though some systems recommend the reaction take place anywhere from room temperatureRoom temperature-Comfort levels:The American Society of Heating, Refrigerating and Air-Conditioning Engineers has listings for suggested temperatures and air flow rates in different types of buildings and different environmental circumstances. For example, a single office in a building has an occupancy ratio per...
to 55 °C (131 °F) for safety reasons. Recommended reaction time varies from 1 to 8 hours; under normal conditions the reaction rate will double with every 10 °C increase in reaction temperature. Excess alcohol is normally used to ensure total conversion of the fat or oil to its esters.
- The glycerin phase is much denser than biodiesel phase and the two can be gravity separated with glycerin simply drawn off the bottom of the settlingSettlingSettling is the process by which particulates settle to the bottom of a liquid and form a sediment. Particles that experience a force, either due to gravity or due to centrifugal motion will tend to move in a uniform manner in the direction exerted by that force...
vessel. In some cases, a centrifugeCentrifugationCentrifugation is a process that involves the use of the centrifugal force for the sedimentation of mixtures with a centrifuge, used in industry and in laboratory settings. More-dense components of the mixture migrate away from the axis of the centrifuge, while less-dense components of the mixture...
is used to separate the two materials faster.
- Once the glycerin and biodiesel phases have been separated, the excess alcohol in each phase is removed with a flash evaporationFlash evaporationFlash evaporation is the partial vapor that occurs when a saturated liquid stream undergoes a reduction in pressure by passing through a throttling valve or other throttling device. This process is one of the simplest unit operations...
process or by distillation. In other systems, the alcohol is removed and the mixture neutralized before the glycerin and esters have been separated. In either case, the alcohol is recovered using distillationDistillationDistillation is a method of separating mixtures based on differences in volatilities of components in a boiling liquid mixture. Distillation is a unit operation, or a physical separation process, and not a chemical reaction....
equipment and is re-used. Care must be taken to ensure no water accumulates in the recovered alcohol stream.
- The glycerin by-product contains unused catalyst and soaps that are neutralized with an acid and sent to storage as crude glycerin (water and alcohol are removed later, chiefly using evaporationEvaporationEvaporation is a type of vaporization of a liquid that occurs only on the surface of a liquid. The other type of vaporization is boiling, which, instead, occurs on the entire mass of the liquid....
, to produce 80-88% pure glycerin).
- Once separated from the glycerin, the biodiesel is sometimes purified by washing gently with warm water to remove residual catalyst or soaps, dried, and sent to storage.
Supercritical process
An alternative, catalyst-free method for transesterification uses supercriticalSupercritical fluid
A supercritical fluid is any substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist. It can effuse through solids like a gas, and dissolve materials like a liquid...
methanol at high temperatures and pressures in a continuous process. In the supercritical state, the oil and methanol are in a single phase, and reaction occurs spontaneously and rapidly. The process can tolerate water in the feedstock, free fatty acids are converted to methyl esters instead of soap, so a wide variety of feedstocks can be used. Also the catalyst removal step is eliminated.
High temperatures and pressures are required, but energy costs of production are similar or less than catalytic production routes.
Ultra- and high-shear in-line and batch reactors
Ultra- and High Shear in-line or batch reactors allow production of biodiesel continuously, semi- continuously, and in batch-mode. This drastically reduces production time and increases production volume.The reaction takes place in the high-energetic shear zone of the Ultra- and High Shear mixer by reducing the droplet size of the immiscible liquids such as oil or fats and methanol. Therefore, the smaller the droplet size the larger the surface area the faster the catalyst can react.
Ultrasonic-reactor method
In the ultrasonic reactor method, the ultrasonic waves cause the reaction mixture to produce and collapse bubbles constantly. This cavitation provides simultaneously the mixing and heating required to carry out the transesterification process. Thus using an ultrasonic reactor for biodiesel production drastically reduces the reaction time, reaction temperatures, and energy input. Hence the process of transesterification can run inline rather than using the time consuming batch processing. Industrial scale ultrasonic devices allow for the industrial scale processing of several thousand barrels per day.Microwave method
Current research is being directed into using commercial microwave ovens to provide the heat needed in the transesterification process. The microwaves provide intense localized heating that may be higher than the recorded temperature of the reaction vessel. A continuous flow process producing 6 liters/minute at a 99% conversion rate has been developed and shown to consume only one-fourth of the energy required in the batch process. Although it is still in the lab-scale, development stage, the microwave method holds great potential to be an efficient and cost-competitive method for commercial-scale biodiesel production.Lipase-catalyzed method
Large amounts of research have focused recently on the use of enzymes as a catalyst for the transesterification. Researchers have found that very good yields could be obtained from crude and used oils using lipaseLipase
A lipase is an enzyme that catalyzes the formation or cleavage of fats . Lipases are a subclass of the esterases.Lipases perform essential roles in the digestion, transport and processing of dietary lipids in most, if not all, living organisms...
s. The use of lipases makes the reaction less sensitive to high FFA content which is a problem with the standard biodiesel process. One problem with the lipase reaction is that methanol cannot be used because it inactivates the lipase catalyst after one batch. However, if methyl acetate is used instead of methanol, the lipase is not in-activated and can be used for several batches, making the lipase system much more cost effective.
Further reading
- Carlson, Kimberly Sue, AH4U, 2004
- Gerpen, J.V., Biodiesel processing and production, Fuel Processing Technology, 2005, 86, 1097-1107. doi:10.1016/j.fuproc.2004.11.005
- Ma, F. & Hanna, M.A., Biodiesel production: a review, Bioresource Technology, 1999, 70, 1-15. doi:10.1016/S0960-8524(99)00025-5
External links
- Fast-Transesterification of Soybean Oil Using Ultrasonication
- Sonochemistry in Transesterification of Biodiesel
- Current State of Ultrasonic Processing for Fast Biodiesel Production
- Biodiesel Production Technology August 2002 - January 2004
- UNL Chemical and Biomolecular Engineering Research and Publications
- Continuous Process for the conversion of vegetable Oils into Methyl Esters of Fatty Acids
- Biodiesel Production Technology
- How to Make Biodiesel
- Analyses of Soybean Biodiesel

