Batch distillation
Encyclopedia
Batch distillation refers to the use of distillation
in batches, meaning that a mixture is distilled to separate it into its component fractions before the distillation still is again charged with more mixture and the process is repeated. This is in contrast with continuous distillation
where the feedstock is added and the distillate drawn off without interruption.
Batch distillation has always been an important part of the production of seasonal, or low capacity and high-purity chemicals. It is a very frequent separation process
in the pharmaceutical industry
and in wastewater
treatment units.
 The simplest and most frequently used batch distillation configuration is the batch rectifier, including the alembic
The simplest and most frequently used batch distillation configuration is the batch rectifier, including the alembic
and pot still
.
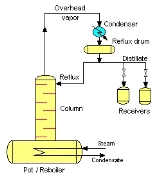
The batch rectifier consists of a pot (or reboiler
), rectifying column, a condenser
, some means of splitting off a portion of the condensed
vapour (distillate) as reflux
, and one or more receivers.
The pot is filled with liquid mixture and heated. Vapour flows upwards in the rectifying column and condenses at the top. Usually, the entire condensate is initially returned to the column as reflux
. This contacting of vapour and liquid considerably improves the separation. Generally, this step is named start-up. The first condensate is the head, and it contains undesirable components. The last condensate is the feints and it is also undesirable, although it adds flavor. In between is the heart and this forms the desired product.
The head and feints may be thrown out, refluxed, or added to the next batch of mash/juice, according to the practice of the distiller. After some time, a part of the overhead condensate is withdrawn continuously as distillate and it is accumulated in the receivers, and the other part is recycled into the column as reflux.
Owing to the differing vapour pressures of the distillate, there will be a change in the overhead distillation with time, as early on in the batch distillation, the distillate will contain a high concentration of the component with the higher relative volatility. As the supply of the material is limited and lighter components are removed, the relative fraction of heavier components will increase as the distillation progresses.
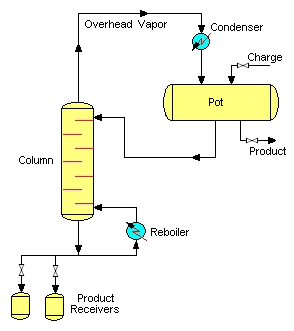 The other simple batch distillation configuration is the batch stripper. The batch stripper consists of the same parts as the batch rectifier. However, in this case, the charge pot is located above the stripping column.
The other simple batch distillation configuration is the batch stripper. The batch stripper consists of the same parts as the batch rectifier. However, in this case, the charge pot is located above the stripping column.
During operation (after charging the pot and starting up the system) the high boiling constituents are primarily separated from the charge mixture. The liquid in the pot is depleted in the high boiling constituents, and enriched in low boiling ones. The high boiling product is routed into the bottom product receivers. The residual low boiling product is withdrawn from the charge pot. This mode of batch distillation is very seldom applied in industrial processes.
During the feasibility studies, the following basic simplifying assumptions are made:
Bernot et al. used the batch distillation regions to determine the sequence of the fractions. According to Ewell and Welch, a batch distillation region gives the same fractions upon rectification of any mixture lying within it. Bernot et al. examined the still and distillate paths for the determination of the region boundaries under high number of stages and high reflux ratio, named maximal separation. Pham and Doherty in pioneering work described the structure and properties of residue curve maps for ternary heterogeneous azeotropic mixtures. In their model, the possibility of the phase separation of the vapour condensed is not taken into consideration yet. The singular points of the residue curve maps determined by this method were used to assign batch distillation regions by Rodriguez-Donis et al. and Skouras et al. Modla et al. pointed out that this method may give misleading results for the minimal amount of entrainer. Lang and Modla extended the method of Pham and Doherty and suggested a new, general method for the calculation of residue curves and for the determination of batch distillation regions of heteroazeotropic distillation.
Lelkes et al. published a feasibility method for the separation of minimum boiling point azeotropes by continuously entrainer feeding batch distillation. This method has been applied for the use of a light entrainer in the batch rectifier and stripper by Lang et al. (1999) and it applied for maximum azeotropes by Lang et al. Modla et al. extended this method for batch heteroazeotropic distillation under continuous entrainer feeding.
Distillation
Distillation is a method of separating mixtures based on differences in volatilities of components in a boiling liquid mixture. Distillation is a unit operation, or a physical separation process, and not a chemical reaction....
in batches, meaning that a mixture is distilled to separate it into its component fractions before the distillation still is again charged with more mixture and the process is repeated. This is in contrast with continuous distillation
Continuous distillation
Continuous distillation, a form of distillation, is an ongoing separation in which a mixture is continuously fed into the process and separated fractions are removed continuously as output streams. A distillation is the separation or partial separation of a liquid feed mixture into components or...
where the feedstock is added and the distillate drawn off without interruption.
Batch distillation has always been an important part of the production of seasonal, or low capacity and high-purity chemicals. It is a very frequent separation process
Separation process
In chemistry and chemical engineering, a separation process, or simply a separation, is any mass transfer process used to convert a mixture of substances into two or more distinct product mixtures, at least one of which is enriched in one or more of the mixture's constituents. In some cases, a...
in the pharmaceutical industry
Pharmaceutical company
The pharmaceutical industry develops, produces, and markets drugs licensed for use as medications. Pharmaceutical companies are allowed to deal in generic and/or brand medications and medical devices...
and in wastewater
Wastewater
Wastewater is any water that has been adversely affected in quality by anthropogenic influence. It comprises liquid waste discharged by domestic residences, commercial properties, industry, and/or agriculture and can encompass a wide range of potential contaminants and concentrations...
treatment units.
Batch rectifier

Alembic
An alembic is an alchemical still consisting of two vessels connected by a tube...
and pot still
Pot still
A pot still is a type of still used in distilling spirits such as whisky or brandy. Heat is applied directly to the pot containing the wash or wine . This is called a batch distillation ....
.
The batch rectifier consists of a pot (or reboiler
Reboiler
Reboilers are heat exchangers typically used to provide heat to the bottom of industrial distillation columns. They boil the liquid from the bottom of a distillation column to generate vapors which are returned to the column to drive the distillation separation....
), rectifying column, a condenser
Condenser (heat transfer)
In systems involving heat transfer, a condenser is a device or unit used to condense a substance from its gaseous to its liquid state, typically by cooling it. In so doing, the latent heat is given up by the substance, and will transfer to the condenser coolant...
, some means of splitting off a portion of the condensed
Condensation
Condensation is the change of the physical state of matter from gaseous phase into liquid phase, and is the reverse of vaporization. When the transition happens from the gaseous phase into the solid phase directly, the change is called deposition....
vapour (distillate) as reflux
Reflux
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations...
, and one or more receivers.
The pot is filled with liquid mixture and heated. Vapour flows upwards in the rectifying column and condenses at the top. Usually, the entire condensate is initially returned to the column as reflux
Reflux
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations...
. This contacting of vapour and liquid considerably improves the separation. Generally, this step is named start-up. The first condensate is the head, and it contains undesirable components. The last condensate is the feints and it is also undesirable, although it adds flavor. In between is the heart and this forms the desired product.
The head and feints may be thrown out, refluxed, or added to the next batch of mash/juice, according to the practice of the distiller. After some time, a part of the overhead condensate is withdrawn continuously as distillate and it is accumulated in the receivers, and the other part is recycled into the column as reflux.
Owing to the differing vapour pressures of the distillate, there will be a change in the overhead distillation with time, as early on in the batch distillation, the distillate will contain a high concentration of the component with the higher relative volatility. As the supply of the material is limited and lighter components are removed, the relative fraction of heavier components will increase as the distillation progresses.
Batch stripper

During operation (after charging the pot and starting up the system) the high boiling constituents are primarily separated from the charge mixture. The liquid in the pot is depleted in the high boiling constituents, and enriched in low boiling ones. The high boiling product is routed into the bottom product receivers. The residual low boiling product is withdrawn from the charge pot. This mode of batch distillation is very seldom applied in industrial processes.

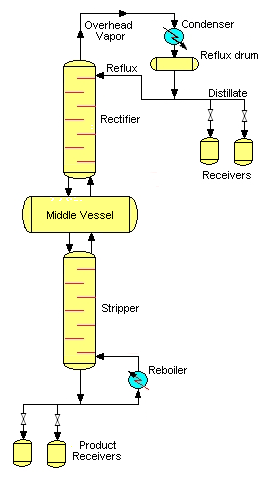
Middle vessel column
A third feasible batch column configuration is the middle vessel column. The middle vessel column consists of both a rectifying and a stripping section and the charge pot is located at the middle of the column.Feasibility studies
Generally, the feasibility studies of batch distillation are based on analyses of the following maps:- Residue Curve map
- still path map
- distillate path map
- different column profile maps
During the feasibility studies, the following basic simplifying assumptions are made:
- infinite number of equilibrium stages
- infinite reflux ratio
- negligible tray hold-up in the two column sections
- quasi-steady state in the column
- constant molar overflow
Bernot et al. used the batch distillation regions to determine the sequence of the fractions. According to Ewell and Welch, a batch distillation region gives the same fractions upon rectification of any mixture lying within it. Bernot et al. examined the still and distillate paths for the determination of the region boundaries under high number of stages and high reflux ratio, named maximal separation. Pham and Doherty in pioneering work described the structure and properties of residue curve maps for ternary heterogeneous azeotropic mixtures. In their model, the possibility of the phase separation of the vapour condensed is not taken into consideration yet. The singular points of the residue curve maps determined by this method were used to assign batch distillation regions by Rodriguez-Donis et al. and Skouras et al. Modla et al. pointed out that this method may give misleading results for the minimal amount of entrainer. Lang and Modla extended the method of Pham and Doherty and suggested a new, general method for the calculation of residue curves and for the determination of batch distillation regions of heteroazeotropic distillation.
Lelkes et al. published a feasibility method for the separation of minimum boiling point azeotropes by continuously entrainer feeding batch distillation. This method has been applied for the use of a light entrainer in the batch rectifier and stripper by Lang et al. (1999) and it applied for maximum azeotropes by Lang et al. Modla et al. extended this method for batch heteroazeotropic distillation under continuous entrainer feeding.
See also
- AlembicAlembicAn alembic is an alchemical still consisting of two vessels connected by a tube...
- AzeotropeAzeotropeAn azeotrope is a mixture of two or more liquids in such a ratio that its composition cannot be changed by simple distillation. This occurs because, when an azeotrope is boiled, the resulting vapor has the same ratio of constituents as the original mixture....
- Azeotropic distillationAzeotropic distillationIn chemistry, azeotropic distillation is any of a range of techniques used to break an azeotrope in distillation. In chemical engineering, azeotropic distillation usually refers to the specific technique of adding another component to generate a new, lower-boiling azeotrope that is heterogeneous...
- Extractive distillationExtractive distillationExtractive distillation is defined as distillation in the presence of a miscible, high boiling, relatively non-volatile component, the solvent, that forms no azeotrope with the other components in the mixture. The method is used for mixtures having a low value of relative volatility, nearing unity...
- Fractional distillationFractional distillationFractional distillation is the separation of a mixture into its component parts, or fractions, such as in separating chemical compounds by their boiling point by heating them to a temperature at which several fractions of the compound will evaporate. It is a special type of distillation...
- HeteroazeotropeHeteroazeotropeA heteroazeotrope is an azeotrope where the vapour phase coexists with two liquid phases.Sketch of a T-x/y equilibrium curve of a typical heteroazeotropic mixture- Examples of heteroazeotropes :*Benzene - Water NBP 69.2 °C...
- Pot stillPot stillA pot still is a type of still used in distilling spirits such as whisky or brandy. Heat is applied directly to the pot containing the wash or wine . This is called a batch distillation ....
- Steam distillationSteam distillationSteam distillation is a special type of distillation for temperature sensitive materials like natural aromatic compounds....
- Vacuum distillationVacuum distillationVacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor pressure causing evaporation of the most volatile liquid...
- Theoretical plateTheoretical plateA theoretical plate in many separation processes is a hypothetical zone or stage in which two phases, such as the liquid and vapor phases of a substance, establish an equilibrium with each other. Such equilibrium stages may also be referred to as an equilibrium stage, ideal stage or a theoretical...
Further reading
- Hilmen Eva-Katrine, Separation of Azeotropic Mixtures:Tools for Analysis and Studies on Batch Distillation Operation, Thesis, Norwegian University of Science and Technology Department of Chemical Engineering, (2000).
External links
- Batch distillation program online Batch distillation of the hydrocarbon compounds.

