
Base excess
Encyclopedia
In human physiology
, base excess and base deficit refer to an excess or deficit, respectively, in the amount of base
present in the blood. The value is usually reported as a concentration in units of mEq/L, with positive numbers indicating an excess of base and negative a deficit. A typical reference range for base excess is −2 to +2 mEq/L.
Comparison of the base excess with the reference range assists in determining whether an acid/base disturbance
is caused by a respiratory, metabolic, or mixed metabolic/respiratory problem. While carbon dioxide
defines the respiratory component of acid-base balance, base excess defines the metabolic component. Accordingly, measurement of base excess is defined under a standardized pressure of carbon dioxide, by titrating
back to a standardized blood pH
of 7.40.
The predominant base contributing to base excess is bicarbonate
. Thus, a deviation of serum bicarbonate from the reference range is ordinarily mirrored by a deviation in base excess. However, base excess is a more comprehensive measurement, encompassing all metabolic contributions.
A further distinction can be made between actual and standard base excess: actual base excess is that present in the blood, while standard base excess is the value when the hemoglobin
is at 5 g/dl. The latter gives a better view of the base excess of the entire extracellular fluid
.
The term and concept of base excess were first introduced by Poul Astrup
and Ole Siggaard-Andersen
in 1958.
concentration ([HCO3-]) and pH
by the equation:
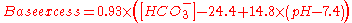
with units of mEq/L. The same can be alternatively expressed as
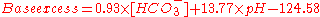
Blood pH is determined by both a metabolic component, measured by base excess, and a respiratory component, measured by pCO2 (partial pressure of carbon dioxide
). Often a disturbance in one triggers a partial compensation in the other. A secondary (compensatory) process can be readily identified because it opposes the observed deviation in blood pH.
For example, inadequate ventilation, a respiratory problem, causes a buildup of CO2, hence respiratory acidosis; the kidneys then compensate by raising blood bicarbonate, producing metabolic alkalosis; such a patient would have a low blood pH, i.e. acidosis, caused by respiratory acidosis and mitigated by compensatory metabolic alkalosis.
A high base excess, thus metabolic alkalosis
, usually involves an excess of bicarbonate
. It can be caused by
A base deficit (a below-normal base excess), thus metabolic acidosis
, usually involves either excretion of bicarbonate or neutralization of bicarbonate by excess organic acids. Common causes include
The serum anion gap
is useful for determining whether a base deficit is caused by addition of acid or loss of bicarbonate.
Physiology
Physiology is the science of the function of living systems. This includes how organisms, organ systems, organs, cells, and bio-molecules carry out the chemical or physical functions that exist in a living system. The highest honor awarded in physiology is the Nobel Prize in Physiology or...
, base excess and base deficit refer to an excess or deficit, respectively, in the amount of base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
present in the blood. The value is usually reported as a concentration in units of mEq/L, with positive numbers indicating an excess of base and negative a deficit. A typical reference range for base excess is −2 to +2 mEq/L.
Comparison of the base excess with the reference range assists in determining whether an acid/base disturbance
Acid-base homeostasis
Acid–base homeostasis is the part of human homeostasis concerning the proper balance between acids and bases, in other words, the pH. The body is very sensitive to its pH level, so strong mechanisms exist to maintain it...
is caused by a respiratory, metabolic, or mixed metabolic/respiratory problem. While carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
defines the respiratory component of acid-base balance, base excess defines the metabolic component. Accordingly, measurement of base excess is defined under a standardized pressure of carbon dioxide, by titrating
Acid-base titration
An acid-base titration is the determination of the concentration of an acid or base by exactly neutralizing the acid/base with an acid or base of known concentration. This allows for quantitative analysis of the concentration of an unknown acid or base solution...
back to a standardized blood pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
of 7.40.
The predominant base contributing to base excess is bicarbonate
Bicarbonate
In inorganic chemistry, bicarbonate is an intermediate form in the deprotonation of carbonic acid...
. Thus, a deviation of serum bicarbonate from the reference range is ordinarily mirrored by a deviation in base excess. However, base excess is a more comprehensive measurement, encompassing all metabolic contributions.
Definition
Base excess is defined as the amount of strong acid that must be added to each liter of fully oxygenated blood to return the pH to 7.40 at a temperature of 37°C and a pCO2 of 40 mmHg (5,332.9 Pa). A base deficit (i.e., a negative base excess) can be correspondingly defined in terms of the amount of strong base that must be added.A further distinction can be made between actual and standard base excess: actual base excess is that present in the blood, while standard base excess is the value when the hemoglobin
Hemoglobin
Hemoglobin is the iron-containing oxygen-transport metalloprotein in the red blood cells of all vertebrates, with the exception of the fish family Channichthyidae, as well as the tissues of some invertebrates...
is at 5 g/dl. The latter gives a better view of the base excess of the entire extracellular fluid
Extracellular fluid
Extracellular fluid usually denotes all body fluid outside of cells. The remainder is called intracellular fluid.In some animals, including mammals, the extracellular fluid can be divided into two major subcompartments, interstitial fluid and blood plasma...
.
The term and concept of base excess were first introduced by Poul Astrup
Poul Bjørndahl Astrup
Poul Bjørndahl Astrup was a Danish clinical chemist famous for inventing a CO2 electrode and co-inventing the concept of base excess.-External links:*...
and Ole Siggaard-Andersen
Ole Siggaard-Andersen
Ole Siggaard-Andersen is a Danish clinical chemist who elucidated much acid base physiology. He jointly invented the concepts of base excess and standard base excess....
in 1958.
Estimation
Base excess can be estimated from the serum bicarbonateBicarbonate
In inorganic chemistry, bicarbonate is an intermediate form in the deprotonation of carbonic acid...
concentration ([HCO3-]) and pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
by the equation:
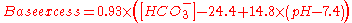
with units of mEq/L. The same can be alternatively expressed as
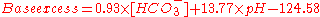
Interpretation
Base excess beyond the reference range indicates- metabolic alkalosisMetabolic alkalosisMetabolic alkalosis is a metabolic condition in which the pH of tissue is elevated beyond the normal range . This is the result of decreased hydrogen ion concentration, leading to increased bicarbonate, or alternatively a direct result of increased bicarbonate concentrations.-Terminology:*Alkalosis...
if too high (more than +2 mEq/L) - metabolic acidosisMetabolic acidosisIn medicine, metabolic acidosis is a condition that occurs when the body produces too much acid or when the kidneys are not removing enough acid from the body. If unchecked, metabolic acidosis leads to acidemia, i.e., blood pH is low due to increased production of hydrogen by the body or the...
if too low (less than −2 mEq/L)
Blood pH is determined by both a metabolic component, measured by base excess, and a respiratory component, measured by pCO2 (partial pressure of carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
). Often a disturbance in one triggers a partial compensation in the other. A secondary (compensatory) process can be readily identified because it opposes the observed deviation in blood pH.
For example, inadequate ventilation, a respiratory problem, causes a buildup of CO2, hence respiratory acidosis; the kidneys then compensate by raising blood bicarbonate, producing metabolic alkalosis; such a patient would have a low blood pH, i.e. acidosis, caused by respiratory acidosis and mitigated by compensatory metabolic alkalosis.
A high base excess, thus metabolic alkalosis
Metabolic alkalosis
Metabolic alkalosis is a metabolic condition in which the pH of tissue is elevated beyond the normal range . This is the result of decreased hydrogen ion concentration, leading to increased bicarbonate, or alternatively a direct result of increased bicarbonate concentrations.-Terminology:*Alkalosis...
, usually involves an excess of bicarbonate
Bicarbonate
In inorganic chemistry, bicarbonate is an intermediate form in the deprotonation of carbonic acid...
. It can be caused by
- Compensation for primary respiratory acidosisRespiratory acidosisRespiratory acidosis is a medical condition in which decreased ventilation causes increased blood carbon dioxide concentration and decreased pH ....
- Excessive loss of HCl in gastric juice by vomiting
- Renal overproduction of bicarbonate, in either contraction alkalosisContraction alkalosisContraction alkalosis refers to the increase in blood pH that occurs as a result of fluid losses . The change in pH is especially pronounced with acidic fluid losses caused by problems like vomiting.-Pathophysiology:...
or Cushing's diseaseCushing's diseaseCushing's disease is a cause of Cushing's Syndrome characterised by increased secretion of adrenocorticotropic hormone from the anterior pituitary. This is most often as a result of a pituitary adenoma...
A base deficit (a below-normal base excess), thus metabolic acidosis
Metabolic acidosis
In medicine, metabolic acidosis is a condition that occurs when the body produces too much acid or when the kidneys are not removing enough acid from the body. If unchecked, metabolic acidosis leads to acidemia, i.e., blood pH is low due to increased production of hydrogen by the body or the...
, usually involves either excretion of bicarbonate or neutralization of bicarbonate by excess organic acids. Common causes include
- Compensation for primary respiratory alkalosisRespiratory alkalosisRespiratory alkalosis is a medical condition in which increased respiration elevates the blood pH...
- Diabetic ketoacidosisDiabetic ketoacidosisDiabetic ketoacidosis is a potentially life-threatening complication in patients with diabetes mellitus. It happens predominantly in those with type 1 diabetes, but it can occur in those with type 2 diabetes under certain circumstances...
, in which high levels of acidic ketone bodiesKetone bodiesKetone bodies are three water-soluble compounds that are produced as by-products when fatty acids are broken down for energy in the liver and kidney. They are used as a source of energy in the heart and brain. In the brain, they are a vital source of energy during fasting...
are produced - Lactic acidosisLactic acidosisLactic acidosis is a physiological condition characterized by low pH in body tissues and blood accompanied by the buildup of lactate especially D-lactate, and is considered a distinct form of metabolic acidosis. The condition typically occurs when cells receive too little oxygen , for example...
, due to anaerobic metabolism during heavy exercise or hypoxiaHypoxia (medical)Hypoxia, or hypoxiation, is a pathological condition in which the body as a whole or a region of the body is deprived of adequate oxygen supply. Variations in arterial oxygen concentrations can be part of the normal physiology, for example, during strenuous physical exercise... - Chronic renal failureChronic renal failureChronic kidney disease , also known as chronic renal disease, is a progressive loss in renal function over a period of months or years. The symptoms of worsening kidney function are unspecific, and might include feeling generally unwell and experiencing a reduced appetite...
, preventing excretion of acid and resorption and production of bicarbonate - DiarrheaDiarrheaDiarrhea , also spelled diarrhoea, is the condition of having three or more loose or liquid bowel movements per day. It is a common cause of death in developing countries and the second most common cause of infant deaths worldwide. The loss of fluids through diarrhea can cause dehydration and...
, in which large amounts of bicarbonate are excreted - Ingestion of poisons such as methanolMethanolMethanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
, ethylene glycolEthylene glycolEthylene glycol is an organic compound widely used as an automotive antifreeze and a precursor to polymers. In its pure form, it is an odorless, colorless, syrupy, sweet-tasting liquid...
, or excessive aspirinAspirinAspirin , also known as acetylsalicylic acid , is a salicylate drug, often used as an analgesic to relieve minor aches and pains, as an antipyretic to reduce fever, and as an anti-inflammatory medication. It was discovered by Arthur Eichengrun, a chemist with the German company Bayer...
The serum anion gap
Anion gap
The anion gap is the difference in the measured cations and the measured anions in serum, plasma, or urine. The magnitude of this difference in the serum is often calculated in medicine when attempting to identify the cause of metabolic acidosis...
is useful for determining whether a base deficit is caused by addition of acid or loss of bicarbonate.
- Base deficit with elevated anion gap indicates addition of acid (e.g., ketoacidosis).
- Base deficit with normal anion gap indicates loss of bicarbonate (e.g., diarrhea). The anion gap is maintained because bicarbonate is exchanged for chlorideChlorideThe chloride ion is formed when the element chlorine, a halogen, picks up one electron to form an anion Cl−. The salts of hydrochloric acid HCl contain chloride ions and can also be called chlorides. The chloride ion, and its salts such as sodium chloride, are very soluble in water...
during excretion.
See also
- Acid-base homeostasisAcid-base homeostasisAcid–base homeostasis is the part of human homeostasis concerning the proper balance between acids and bases, in other words, the pH. The body is very sensitive to its pH level, so strong mechanisms exist to maintain it...
- Metabolic acidosisMetabolic acidosisIn medicine, metabolic acidosis is a condition that occurs when the body produces too much acid or when the kidneys are not removing enough acid from the body. If unchecked, metabolic acidosis leads to acidemia, i.e., blood pH is low due to increased production of hydrogen by the body or the...
/ Metabolic alkalosisMetabolic alkalosisMetabolic alkalosis is a metabolic condition in which the pH of tissue is elevated beyond the normal range . This is the result of decreased hydrogen ion concentration, leading to increased bicarbonate, or alternatively a direct result of increased bicarbonate concentrations.-Terminology:*Alkalosis... - Arterial blood gasArterial blood gasAn arterial blood gas is a blood test that is performed using blood from an artery. It involves puncturing an artery with a thin needle and syringe and drawing a small volume of blood. The most common puncture site is the radial artery at the wrist, but sometimes the femoral artery in the groin or...

