Barton-McCombie deoxygenation
Encyclopedia
The Barton–McCombie deoxygenation is an organic reaction
in which an hydroxy
functional group
in an organic compound
is replaced by a hydride
to give an alkyl group
. It is named for the British chemists Sir Derek Harold Richard Barton
(1918–1998) and Stuart W. McCombie.
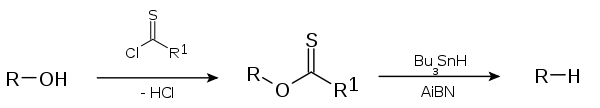 This deoxygenation reaction is a radical substitution
This deoxygenation reaction is a radical substitution
. In the related Barton decarboxylation
the reactant is a carboxylic acid
.
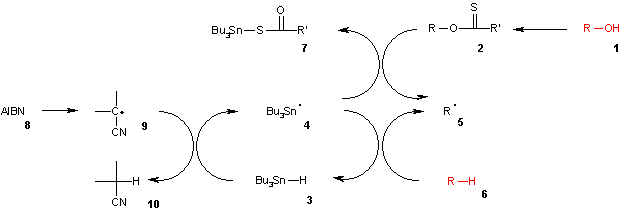
consists of a catalytic radical initiation step and a propagation step. The alcohol
(1) is first converted into a xanthate
(2). The other reactant tributyltin hydride
3 is decomposed by AIBN 8 into a tributyltin radical
4. The tributyltin radical abstracts the xanthate group from 2 leaving an alkyl radical 5 and tributyltin xanthate (7). The sulfur
tin
bond in this compound is very stable and provides the driving force
for this reaction. The alkyl radical in turn abstracts a hydrogen atom from a new molecule of tributyltin hydride generating the desired deoxygenated product (6) and a new radical species ready for propagation.
as the radical source and poly(methylhydridesiloxane)
(PMHS) as the hydride
source . Phenyl chlorothionoformate used as the starting material ultimately generates carbonyl sulfide
.

-water complexes such as trimethylborane
contaminated with small amounts of water.
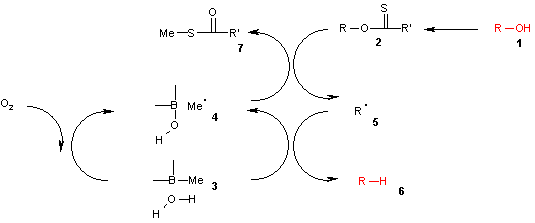
 In this catalytic cycle
In this catalytic cycle
the reaction is initiated by air oxidation of the trialkylborane 3 by air to the methyl radical 4. This radical reacts with the xanthate 2 to S-methyl-S-methyl dithiocarbonate 7 and the radical intermediate 5. The (CH3)3B.H2O complex 3 provides a hydrogen for recombining with this radical to the alkane 6 leaving behind diethyl borinic acid and a new methyl radical.
 It is found by theoretical calculations
It is found by theoretical calculations
that that a O-H homolysis
reaction in the borane-water complex is endothermic with an energy similar to that of the homolysis reaction in tributyltin hydride but much lower than the homolysis reaction of pure water.
of azadirachtin
:
In another variation the reagent is the imidazole
1,1'-thiocarbonyldiimidazole (TCDI), for example in the total synthesis of pallescensin B.. TCDI is especially good to primary alcohols because there is no resonance stabilization of the xanthate because the nitrogen lonepair is involved in the aromatic sextet.
The reaction also applies to S-alkylxanthates. With triethylborane
as a novel metal-free reagent, the required hydrogen atoms are abstracted from protic solvents, the reactor wall or even (in strictly anhydrous conditions) the borane itself.
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
in which an hydroxy
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
in an organic compound
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
is replaced by a hydride
Hydride
In chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties. In compounds that are regarded as hydrides, hydrogen is bonded to a more electropositive element or group...
to give an alkyl group
Alkane
Alkanes are chemical compounds that consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds without any cycles...
. It is named for the British chemists Sir Derek Harold Richard Barton
Derek Harold Richard Barton
Sir Derek Harold Richard Barton FRS was a British organic chemist and Nobel Prize laureate.-Biography:Barton was born to William Thomas and Maude Henrietta Barton. He attended Tonbridge School and in 1938 he entered Imperial College London, where he graduated in 1940 and obtained his Ph.D. degree...
(1918–1998) and Stuart W. McCombie.

Radical substitution
In organic chemistry, a radical substitution reaction is a substitution reaction involving free radicals as a reactive intermediate.The reaction always involves at least two steps, and possibly a third....
. In the related Barton decarboxylation
Barton decarboxylation
The Barton decarboxylation is a radical reaction in which a carboxylic acid is first converted to a thiohydroxamate ester . The product is then heated in the presence of a radical initiator and a suitable hydrogen donor to complete the reductive decarboxylation of the initial carboxylic acid...
the reactant is a carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
.
Mechanism
The reaction mechanismReaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
consists of a catalytic radical initiation step and a propagation step. The alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
(1) is first converted into a xanthate
Xanthate
Xanthate usually refers to a salt with the formula ROCS2-M+ . The name xanthates is derived from Greek ξανθός , meaning “yellowish, golden”, and indeed most xanthate salts are yellow...
(2). The other reactant tributyltin hydride
Organotin
Organotin compounds or stannanes are chemical compounds based on tin with hydrocarbon substituents. Organotin chemistry is part of the wider field of organometallic chemistry. The first organotin compound was diethyltin diiodide, discovered by Edward Frankland in 1849...
3 is decomposed by AIBN 8 into a tributyltin radical
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
4. The tributyltin radical abstracts the xanthate group from 2 leaving an alkyl radical 5 and tributyltin xanthate (7). The sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
tin
Tin
Tin is a chemical element with the symbol Sn and atomic number 50. It is a main group metal in group 14 of the periodic table. Tin shows chemical similarity to both neighboring group 14 elements, germanium and lead and has two possible oxidation states, +2 and the slightly more stable +4...
bond in this compound is very stable and provides the driving force
Standard enthalpy change of reaction
The standard enthalpy of reaction is the enthalpy change that occurs in a system when one mole of matter is transformed by a chemical reaction under standard conditions.For a generic chemical reaction...
for this reaction. The alkyl radical in turn abstracts a hydrogen atom from a new molecule of tributyltin hydride generating the desired deoxygenated product (6) and a new radical species ready for propagation.

Alternative hydride sources
Main disadvantage of this reaction is the use of the tin hydride which is toxic, expensive and difficult to remove from the reaction mixture. One alternative is the use of tributyltin oxideTributyltin oxide
Tributyltin oxide , or bisoxide, is an organotin compound chiefly used as a biocide , especially a wood preservative. Its chemical formula is C24H54OSn2. It has the form of a thin, colorless to pale yellow liquid with melting point -45 °C, boiling point 180 °C, and slight water solubility...
as the radical source and poly(methylhydridesiloxane)
PMHS
Polymethylhydrosiloxane is a polymer with the general structure --. It is used in organic chemistry as a mild and stable reducing agent easily transferring hydrides to metal centers....
(PMHS) as the hydride
Hydride
In chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties. In compounds that are regarded as hydrides, hydrogen is bonded to a more electropositive element or group...
source . Phenyl chlorothionoformate used as the starting material ultimately generates carbonyl sulfide
Carbonyl sulfide
Carbonyl sulfide is the chemical compound with the formula OCS. Commonly written as COS, it is a colourless flammable gas with an unpleasant odor. It is a linear molecule consisting of a carbonyl group double bonded to a sulfur atom...
.

Trialkyl boranes
An even more convenient hydrogen donor is provided by trialkylboraneOrganoborane
Organoborane or organoboron compounds are chemical compounds that are organic derivatives of BH3, for example trialkyl boranes. Organoboron chemistry or organoborane chemistry is the chemistry of these compounds...
-water complexes such as trimethylborane
Trimethylborane
Trimethylborane is a toxic compound normally occurring as a gas that spontaneously catches fire in air. The formula is B3, which can also be expressed as Me3B, with Me representing methyl. Its melting point is -161.5 °C and boiling point is -20.2 °C.Vapour pressure is given by log P =...
contaminated with small amounts of water.

Catalytic cycle
A catalytic cycle in chemistry is a term for a multistep reaction mechanism that involves a catalyst . The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, materials science, etc. Often such cycles show the conversion of a...
the reaction is initiated by air oxidation of the trialkylborane 3 by air to the methyl radical 4. This radical reacts with the xanthate 2 to S-methyl-S-methyl dithiocarbonate 7 and the radical intermediate 5. The (CH3)3B.H2O complex 3 provides a hydrogen for recombining with this radical to the alkane 6 leaving behind diethyl borinic acid and a new methyl radical.

Computational chemistry
Computational chemistry is a branch of chemistry that uses principles of computer science to assist in solving chemical problems. It uses the results of theoretical chemistry, incorporated into efficient computer programs, to calculate the structures and properties of molecules and solids...
that that a O-H homolysis
Homolysis
In general it means breakdown to equal pieces There are separate meanings for the word in chemistry and biology.-Homolysis in chemistry:...
reaction in the borane-water complex is endothermic with an energy similar to that of the homolysis reaction in tributyltin hydride but much lower than the homolysis reaction of pure water.
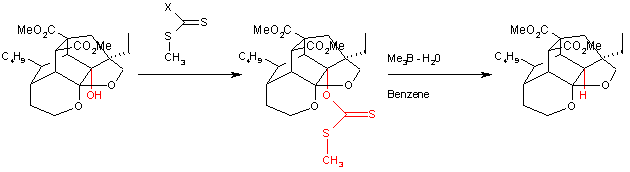
Scope
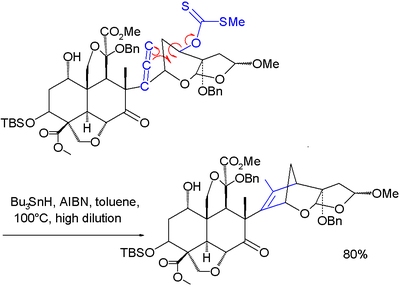
A variation of this reaction was used as one of the steps in the total synthesisTotal synthesis
In organic chemistry, a total synthesis is, in principle, the complete chemical synthesis of complex organic molecules from simpler pieces, usually without the aid of biological processes. In practice, these simpler pieces are commercially available in bulk and semi-bulk quantities, and are often...
of azadirachtin
Azadirachtin
Azadirachtin, a chemical compound belonging to the limonoid group, is a secondary metabolite present in neem seeds. It is a highly oxidized tetranortriterpenoid which boasts a plethora of oxygen functionality, comprising an enol ether, acetal, hemiacetal, and tetra-substituted oxirane as well as a...
:
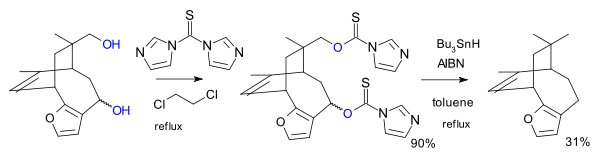
In another variation the reagent is the imidazole
Imidazole
Imidazole is an organic compound with the formula C3H4N2. This aromatic heterocyclic is a diazole and is classified as an alkaloid. Imidazole refers to the parent compound, whereas imidazoles are a class of heterocycles with similar ring structure, but varying substituents...
1,1'-thiocarbonyldiimidazole (TCDI), for example in the total synthesis of pallescensin B.. TCDI is especially good to primary alcohols because there is no resonance stabilization of the xanthate because the nitrogen lonepair is involved in the aromatic sextet.
The reaction also applies to S-alkylxanthates. With triethylborane
Triethylborane
Triethylborane , also called triethylborine and triethylboron, is an organoborane , a near-colorless to yellowish transparent liquid with pungent ether-like odor. Its chemical formula can be written as C6H15B, or 3B, or 3B, or Et3B.Triethylborane is strongly pyrophoric, igniting spontaneously in...
as a novel metal-free reagent, the required hydrogen atoms are abstracted from protic solvents, the reactor wall or even (in strictly anhydrous conditions) the borane itself.
External links
- Barton-McCombie @ organic-chemistry.org
- Chemical & Engineering NewsChemical & Engineering NewsChemical & Engineering News is a weekly magazine published by the American Chemical Society, providing professional and technical information in the fields of chemistry and chemical engineering...
article on alkylborane reaction