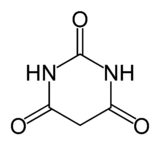
Barbituric acid
Encyclopedia
Barbituric acid or malonylurea or 6-hydroxyuracil is an organic compound
based on a pyrimidine
heterocyclic skeleton. It is an odorless powder soluble in water. Barbituric acid is the parent compound of barbiturate
drugs, although barbituric acid itself is not pharmacologically active. The compound was discovered by the German chemist Adolf von Baeyer
on December 4, 1864, the feast of Saint Barbara
(who gave the compound its namesake), by combining urea
and malonic acid
in a condensation reaction
. Malonic acid has since been replaced by diethyl malonate
, as using the ester avoids the problem of having to deal with the acidity of the carboxylic acid
and its unreactive carboxylate.
The α-carbon has a reactive hydrogen atom and is quite acidic (pKa = 4.01) even for a diketone
species (cf. dimedone
with pKa 5.23 and acetylacetone
with pKa 8.95) because of the additional aromatic stabilisation of the carbanion. Using the Knoevenagel condensation
reaction, barbituric acid can form a large variety of barbiturate
drugs that behave as central nervous system depressants.
Barbituric acid is used in synthesis of riboflavin
.
As of 2007, more than 2550 barbiturates and related compounds have been synthesised, with 50 to 55 in clinical use around the world at present. The first to be used in medicine was barbital
(Veronal) starting in 1903, and the second, phenobarbitone a.k.a. phenobarbital
was first marketed in 1912.
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
based on a pyrimidine
Pyrimidine
Pyrimidine is a heterocyclic aromatic organic compound similar to benzene and pyridine, containing two nitrogen atoms at positions 1 and 3 of the six-member ring...
heterocyclic skeleton. It is an odorless powder soluble in water. Barbituric acid is the parent compound of barbiturate
Barbiturate
Barbiturates are drugs that act as central nervous system depressants, and can therefore produce a wide spectrum of effects, from mild sedation to total anesthesia. They are also effective as anxiolytics, as hypnotics, and as anticonvulsants...
drugs, although barbituric acid itself is not pharmacologically active. The compound was discovered by the German chemist Adolf von Baeyer
Adolf von Baeyer
Johann Friedrich Wilhelm Adolf von Baeyer was a German chemist who synthesized indigo, and was the 1905 recipient of the Nobel Prize in Chemistry. Born in Berlin, he initially studied mathematics and physics at Berlin University before moving to Heidelberg to study chemistry with Robert Bunsen...
on December 4, 1864, the feast of Saint Barbara
Saint Barbara
Saint Barbara, , Feast Day December 4, known in the Eastern Orthodox Church as the Great Martyr Barbara, was an early Christian saint and martyr....
(who gave the compound its namesake), by combining urea
Urea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
and malonic acid
Malonic acid
Malonic acid is a dicarboxylic acid with structure CH22. The ionised form of malonic acid, as well as its esters and salts, are known as malonates. For example, diethyl malonate is malonic acid's ethyl ester...
in a condensation reaction
Condensation reaction
A condensation reaction is a chemical reaction in which two molecules or moieties combine to form one single molecule, together with the loss of a small molecule. When this small molecule is water, it is known as a dehydration reaction; other possible small molecules lost are hydrogen chloride,...
. Malonic acid has since been replaced by diethyl malonate
Diethyl malonate
Diethyl malonate, also known as DEM, is the diethyl ester of malonic acid. It occurs naturally in grapes and strawberries as a colourless liquid with an apple-like odour, and is used in perfumes...
, as using the ester avoids the problem of having to deal with the acidity of the carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
and its unreactive carboxylate.
The α-carbon has a reactive hydrogen atom and is quite acidic (pKa = 4.01) even for a diketone
Diketone
A diketone is a molecule containing two ketone groups. The simpliest diketone is diacetyl, also known as 2,3-butanedione. Diacetyl, acetylacetone, and hexane-2,5-dione are examples of 1,2-, 1,3-, and 1,4-diketones, respectively...
species (cf. dimedone
Dimedone
Dimedone is a cyclic diketone used in organic chemistry to determine whether a compound contains an aldehyde group. Cyclohexanediones in general can be used as catalysts in the formation of transition-metal complexes. Other uses include applications in colourimetry, crystallography, luminescence...
with pKa 5.23 and acetylacetone
Acetylacetone
Acetylacetone is an organic compound that famously exists in two tautomeric forms that rapidly interconvert. The less stable tautomer is a diketone formally named pentane-2,4-dione. The more common tautomer is the enol form. The pair of tautomers rapidly interconvert and are treated as a single...
with pKa 8.95) because of the additional aromatic stabilisation of the carbanion. Using the Knoevenagel condensation
Knoevenagel condensation
The Knoevenagel condensation reaction is an organic reaction named after Emil Knoevenagel. It is a modification of the Aldol condensation.A Knoevenagel condensation is a nucleophilic addition of an active hydrogen compound to a carbonyl group followed by a dehydration reaction in which a molecule...
reaction, barbituric acid can form a large variety of barbiturate
Barbiturate
Barbiturates are drugs that act as central nervous system depressants, and can therefore produce a wide spectrum of effects, from mild sedation to total anesthesia. They are also effective as anxiolytics, as hypnotics, and as anticonvulsants...
drugs that behave as central nervous system depressants.
Barbituric acid is used in synthesis of riboflavin
Riboflavin
Riboflavin, also known as vitamin B2 or additive E101, is an easily absorbed micronutrient with a key role in maintaining health in humans and animals. It is the central component of the cofactors FAD and FMN, and is therefore required by all flavoproteins. As such, vitamin B2 is required for a...
.
As of 2007, more than 2550 barbiturates and related compounds have been synthesised, with 50 to 55 in clinical use around the world at present. The first to be used in medicine was barbital
Barbital
Barbital , also called barbitone, was the first commercially marketed barbiturate. It was used as a sleeping aid from 1903 until the mid-1950s. The chemical names for barbital are diethylmalonyl urea or diethylbarbituric acid...
(Veronal) starting in 1903, and the second, phenobarbitone a.k.a. phenobarbital
Phenobarbital
Phenobarbital or phenobarbitone is a barbiturate, first marketed as Luminal by Friedr. Bayer et comp. It is the most widely used anticonvulsant worldwide, and the oldest still commonly used. It also has sedative and hypnotic properties but, as with other barbiturates, has been superseded by the...
was first marketed in 1912.

