
Avogadro's law
Encyclopedia
Avogadro's law is a gas law named after Amedeo Avogadro
who, in 1811, hypothesized that two given samples of an ideal gas
, at the same temperature
, pressure
and volume
, contain the same number of molecule
s. Thus, the number of molecules or atoms in a specific volume of gas
is independent of their size or the molar mass
of the gas.
As an example, equal volumes of molecular hydrogen
and nitrogen
contain the same number of molecules when they are at the same temperature and pressure, and observe ideal gas
behavior. In practice, real gases show small deviations from the ideal behavior and the law holds only approximately, but is still a useful approximation for scientists.

Where:
The most significant consequence of Avogadro's law is that the ideal gas constant
has the same value for all gases. This means that: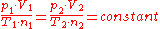
Where:
be the proportionality constant, and rearranging as follows:
This equation is known as the ideal gas law
.
and 273.15 K, we can find the volume of one mole of a gas:
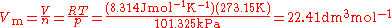
For 100.000 kPa
and 273.15 K, the molar volume of an ideal gas is 22.414 dm3mol-1.
Amedeo Avogadro
Lorenzo Romano Amedeo Carlo Avogadro di Quaregna e di Cerreto, Count of Quaregna and Cerreto was an Italian savant. He is most noted for his contributions to molecular theory, including what is known as Avogadro's law...
who, in 1811, hypothesized that two given samples of an ideal gas
Ideal gas
An ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
, at the same temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
, pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
and volume
Volume
Volume is the quantity of three-dimensional space enclosed by some closed boundary, for example, the space that a substance or shape occupies or contains....
, contain the same number of molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s. Thus, the number of molecules or atoms in a specific volume of gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
is independent of their size or the molar mass
Molar mass
Molar mass, symbol M, is a physical property of a given substance , namely its mass per amount of substance. The base SI unit for mass is the kilogram and that for amount of substance is the mole. Thus, the derived unit for molar mass is kg/mol...
of the gas.
As an example, equal volumes of molecular hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
and nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
contain the same number of molecules when they are at the same temperature and pressure, and observe ideal gas
Ideal gas
An ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
behavior. In practice, real gases show small deviations from the ideal behavior and the law holds only approximately, but is still a useful approximation for scientists.
Mathematical definition
Avogadro's law is stated mathematically as:
Where:
- V is the volume of the gas.
- n is the amount of substanceAmount of substanceAmount of substance is a standards-defined quantity that measures the size of an ensemble of elementary entities, such as atoms, molecules, electrons, and other particles. It is sometimes referred to as chemical amount. The International System of Units defines the amount of substance to be...
of the gas. - k is a proportionality constantPhysical constantA physical constant is a physical quantity that is generally believed to be both universal in nature and constant in time. It can be contrasted with a mathematical constant, which is a fixed numerical value but does not directly involve any physical measurement.There are many physical constants in...
.
The most significant consequence of Avogadro's law is that the ideal gas constant
Gas constant
The gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,...
has the same value for all gases. This means that:
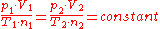
Where:
- p is the pressurePressurePressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
of the gas - T is the temperatureTemperatureTemperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
in kelvinKelvinThe kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
of the gas
Ideal gas law
A common rearrangement of this equation is by letting RGas constant
The gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,...
be the proportionality constant, and rearranging as follows:

This equation is known as the ideal gas law
Ideal gas law
The ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. It was first stated by Émile Clapeyron in 1834 as a combination of Boyle's law and Charles's law...
.
Molar volume
Taking STP to be 101.325 kPaKPA
KPA may refer to:* Kenya Ports Authority* Kiln phosphoric acid, a dry process to produce phosphoric acid at high temperature in a kiln* Kilopascal , a unit of pressure* Known-plaintext attack, a method of cryptanalysis* Korean People's Army...
and 273.15 K, we can find the volume of one mole of a gas:
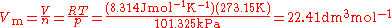
For 100.000 kPa
KPA
KPA may refer to:* Kenya Ports Authority* Kiln phosphoric acid, a dry process to produce phosphoric acid at high temperature in a kiln* Kilopascal , a unit of pressure* Known-plaintext attack, a method of cryptanalysis* Korean People's Army...
and 273.15 K, the molar volume of an ideal gas is 22.414 dm3mol-1.
See also
- Boyle's lawBoyle's lawBoyle's law is one of many gas laws and a special case of the ideal gas law. Boyle's law describes the inversely proportional relationship between the absolute pressure and volume of a gas, if the temperature is kept constant within a closed system...
- Charles's lawCharles's lawCharles' law is an experimental gas law which describes how gases tend to expand when heated. It was first published by French natural philosopher Joseph Louis Gay-Lussac in 1802, although he credited the discovery to unpublished work from the 1780s by Jacques Charles...
- Combined gas lawCombined gas lawThe combined gas law is a gas law which combines Charles's law, Boyle's law, and Gay-Lussac's law. These laws each relate one thermodynamic variable to another mathematically while holding everything else constant. Charles's law states that volume and temperature are directly proportional to each...
- Gay-Lussac's lawGay-Lussac's lawThe expression Gay-Lussac's law is used for each of the two relationships named after the French chemist Joseph Louis Gay-Lussac and which concern the properties of gases, though it is more usually applied to his law of combining volumes, the first listed here...
- Ideal gasIdeal gasAn ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
External links
- Avogadro's law at the University of Fribourg
- Avogadro's law at the Royal Society of Chemistry

