
Arrhenius plot
Encyclopedia
An Arrhenius plot displays the logarithm of kinetic constants (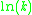 , ordinate
, ordinate
axis) plotted against inverse temperature ( , abscissa
, abscissa
). Arrhenius plots are often used to analyze the effect of temperature on the rates of chemical reactions. For a single rate-limited thermally activated process, an Arrhenius plot gives a straight line, from which the activation energy
and the pre-exponential factor
can both be determined.
The Arrhenius equation
can be given in the form:
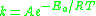
or alternatively
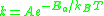
The only difference is the energy units: the former form uses energy/mole
, which is common
in chemistry, while the latter form uses energy directly, which is common in physics.
The different units are accounted for in using either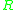 = Gas constant
= Gas constant
or Boltzmanns constant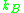 .
.
The former form can be written equivalently as:
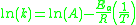
When plotted in the manner described above, the value of the "y-intercept" will correspond to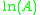 , and the gradient of the line will be equal to
, and the gradient of the line will be equal to 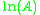 .
.
The pre-exponential factor, A, is a constant of proportionality that takes into account a number of factors such as the frequency of collision between and the orientation of the reacting particles.
The expression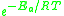 represents the fraction of the molecules present in a gas which have energies equal to or in excess of activation energy at a particular temperature.
represents the fraction of the molecules present in a gas which have energies equal to or in excess of activation energy at a particular temperature.
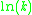 , ordinate
, ordinateOrdinate
In mathematics, ordinate refers to that element of an ordered pair which is plotted on the vertical axis of a two-dimensional Cartesian coordinate system, as opposed to the abscissa...
axis) plotted against inverse temperature (
 , abscissa
, abscissaAbscissa
In mathematics, abscissa refers to that element of an ordered pair which is plotted on the horizontal axis of a two-dimensional Cartesian coordinate system, as opposed to the ordinate...
). Arrhenius plots are often used to analyze the effect of temperature on the rates of chemical reactions. For a single rate-limited thermally activated process, an Arrhenius plot gives a straight line, from which the activation energy
Activation energy
In chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
and the pre-exponential factor
Pre-exponential factor
In chemical kinetics, the preexponential factor or A factor is the pre-exponential constant in the Arrhenius equation, an empirical relationship between temperature and rate coefficient...
can both be determined.
| Example: Nitrogen dioxide decay |
|---|
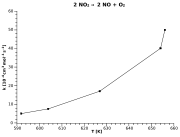 |
 |
The Arrhenius equation
Arrhenius equation
The Arrhenius equation is a simple, but remarkably accurate, formula for the temperature dependence of the reaction rate constant, and therefore, rate of a chemical reaction. The equation was first proposed by the Dutch chemist J. H. van 't Hoff in 1884; five years later in 1889, the Swedish...
can be given in the form:
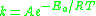
or alternatively
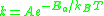
The only difference is the energy units: the former form uses energy/mole
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
, which is common
in chemistry, while the latter form uses energy directly, which is common in physics.
The different units are accounted for in using either
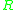 = Gas constant
= Gas constantGas constant
The gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,...
or Boltzmanns constant
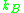 .
.The former form can be written equivalently as:
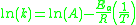
-
- Where:
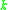 = Rate constant
= Rate constant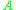 = Pre-exponential factorPre-exponential factorIn chemical kinetics, the preexponential factor or A factor is the pre-exponential constant in the Arrhenius equation, an empirical relationship between temperature and rate coefficient...
= Pre-exponential factorPre-exponential factorIn chemical kinetics, the preexponential factor or A factor is the pre-exponential constant in the Arrhenius equation, an empirical relationship between temperature and rate coefficient...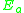 = Activation energyActivation energyIn chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
= Activation energyActivation energyIn chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...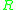 = Gas constantGas constantThe gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,...
= Gas constantGas constantThe gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,...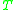 = Absolute temperature, K
= Absolute temperature, K
- Where:
When plotted in the manner described above, the value of the "y-intercept" will correspond to
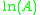 , and the gradient of the line will be equal to
, and the gradient of the line will be equal to 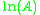 .
.The pre-exponential factor, A, is a constant of proportionality that takes into account a number of factors such as the frequency of collision between and the orientation of the reacting particles.
The expression
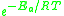 represents the fraction of the molecules present in a gas which have energies equal to or in excess of activation energy at a particular temperature.
represents the fraction of the molecules present in a gas which have energies equal to or in excess of activation energy at a particular temperature.

