
Antithrombin
Encyclopedia
Antithrombin is a small protein molecule that inactivates several enzymes of the coagulation
system. Antithrombin is a glycoprotein
produced by the liver
and consists of 432 amino acids. It contains three disulfide bond
s and a total of four possible glycosylation
sites. α-Antithrombin is the dominant form of antithrombin found in blood plasma
and has an oligosaccharide occupying each of its four glycosylation sites. A single glycosylation site remains consistently un-occupied in the minor form of antithrombin, β-antithrombin. Its activity is increased manifold by the anticoagulant
drug heparin
, which enhances the binding of antithrombin to factor II
and factor X
.
Antithrombin I (AT I) refers to the absorption of thrombin
onto fibrin
after thrombin has activated fibrinogen
. Antithrombin II (AT II) refers to a cofactor in plasma, which together with heparin
interferes with the interaction of thrombin
and fibrinogen
. Antithrombin III (AT III) refers to a substance in plasma
that inactivates thrombin. Antithrombin IV (AT IV) refers to an antithrombin that becomes activated during and shortly after blood coagulation. Only AT III and possibly AT I are medically significant. AT III is generally referred to solely as "Antithrombin" and it is Antithrombin III that is discussed in this article.
 Antithrombin has a half-life
Antithrombin has a half-life
in blood plasma
of around 3 days.
The normal antithrombin concentration in human blood plasma
is high at approximately 0.12 mg/ml, which is equivalent to a molar concentration of 2.3 μM.
Antithrombin has been isolated from the plasma of a large number of species additional to humans. As deduced from protein and cDNA sequencing, cow, sheep, rabbit and mouse antithrombins are all 433 amino acids in length, which is one amino acid longer than human antithrombin. The extra amino acid is thought to occur at amino acid position 6. Cow, sheep, rabbit, mouse, and human antithrombins share between 84 and 89% amino acid sequence identity. Six of the amino acids form three intramolecular disulfide bond
s, Cys
8-Cys128, Cys21-Cys95, and Cys248-Cys430.
They all have four potential N-glycosylation sites. These occur at asparagine
(Asn) amino acid numbers 96, 135, 155, and 192 in humans and at similar amino acid numbers in other species. All these sites are occupied by covalently attached oligosaccharide side-chains in the predominant form of human antithrombin, α-antithrombin, resulting in a molecular weight for this form of antithrombin of 58,200. The potential glycosylation site at asparagine 135 is not occupied in a minor form (around 10%) of antithrombin, β-antithrombin (see Figure 1).
Recombinant
antithrombins with properties similar to those of normal human antithrombin have been produced using baculovirus
-infected insect cells and mammalian cell lines grown in cell culture
. These recombinant antithrombins generally have different glycosylation patterns to normal antithrombin and are typically used in antithrombin structural studies. For this reason many of the antithrombin structures stored in the protein data bank
and presented in this article show variable glycosylation patterns.
Antithrombin begins in its native state, which has a higher free energy compared to the latent state, which it decays to on average after 3 days. The latent state has the same form as the activated state - that is, when it is inhibiting thrombin. As such it is a classic example of the utility of kinetic vs thermodynamic control of protein folding.

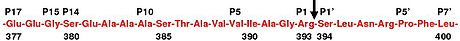 Antithrombin is a serpin (serine protease inhibitor) and is thus similar in structure to most other plasma
Antithrombin is a serpin (serine protease inhibitor) and is thus similar in structure to most other plasma
protease
inhibitor
s, such as alpha 1-antichymotrypsin
, alpha 2-antiplasmin
and Heparin cofactor II
.
The physiological target protease
s of antithrombin are those of the contact activation pathway (formerly known as the intrinsic pathway), namely the activated forms of Factor X
(Xa), Factor IX
(IXa), Factor XI
(XIa), Factor XII
(XIIa) and, to a greater extent, Factor II (thrombin) (IIa), and also the activated form of Factor VII
(VIIa) from the tissue factor pathway (formerly known as the extrinsic pathway). The inhibitor also inactivates kallikrein
and plasmin
, also involved in blood coagulation. However it inactivates certain other serine proteases that are not involved in coagulation such as trypsin
and the C1s subunit of the enzyme C1 involved in the classical complement pathway
.
Protease inactivation results as a consequence of trapping the protease in an equimolar complex with antithrombin in which the active site of the protease enzyme is inaccessible to its usual substrate
. The formation of an antithrombin-protease complex involves an interaction between the protease and a specific reactive peptide bond
within antithrombin. In human antithrombin this bond is between arginine
(arg) 393 and serine
(ser) 394 (see Figure 2 and Figure 3).
It is thought that protease enzymes become trapped in inactive antithrombin-protease complexes as a consequence of their attack on the reactive bond. Although attacking a similar bond within the normal protease substrate results in rapid proteolytic cleavage of the substrate, initiating an attack on the antithrombin reactive bond causes antithrombin to become activated and trap the enzyme at an intermediate stage of the proteolytic process. Given time, thrombin is able to cleave the reactive bond within antithrombin and an inactive antithrombin-thrombin complex will dissociate, however the time it takes for this to occur may be greater than 3 days. However bonds P3-P4 and P1'-P2' can be rapidly cleaved by neutrophil elastase and the bacterial enzyme thermolysin
respectively, resulting in inactive antithrombins no longer able to inhibit thrombin activity.
The rate of antithrombin's inhibition of protease activity is greatly enhanced by its additional binding to heparin
as is its inactivation by neutrophil elastase.
.
AT-III binds to a specific pentasaccharide sulfation sequence contained within the heparin polymer
GlcNAc/NS(6S)-GlcA-GlcNS(3S,6S)-IdoA(2S)-GlcNS(6S)
Upon binding to this pentasaccharide sequence, inhibition of protease activity is increased by heparin as a result of two distinct mechanisms. In one mechanism heparin stimulation of Factor IXa and Xa inhibition depends on a conformational change within antithrombin involving the reactive site loop and is thus allosteric. In another mechanism stimulation of thrombin inhibition depends on the formation of a ternary complex
between AT-III, thrombin, and heparin.
 Increased Factor IXa and Xa inhibition requires the minimal heparin pentasaccharide sequence. The conformational changes that occur within antithrombin in response to pentasaccharide binding are well documented.
Increased Factor IXa and Xa inhibition requires the minimal heparin pentasaccharide sequence. The conformational changes that occur within antithrombin in response to pentasaccharide binding are well documented.
In the absence of heparin, amino acids P14 and P15 (see Figure 3) from the reactive site loop are embedded within the main body of the protein (specifically the top of beta sheet
A). This feature is in common with other serpin
s such as heparin cofactor II
, alpha 1-antichymotrypsin
and MENT
.
The conformational change most relevant for Factor IXa and Xa inhibition involves the P14 and P15 amino acids within the N-terminal region of the reactive site loop (circled in Figure 4 model B). This region has been termed the hinge region. The conformational change within the hinge region in response to heparin binding results in the expulsion of P14 and P15 from the main body of the protein and it has been shown that by preventing this conformational change, increased Factor IXa and Xa inhibition does not occur. It is thought that the increased flexibility given to the reactive site loop as a result of the hinge region conformational change is a key factor in influencing increased Factor IXa and Xa inhibition. It has been calculated that in the absence of the pentasaccharide only one in every 400 Antithrombin molecules (0.25%) is in an active conformation with the P14 and P15 amino acids expelled.
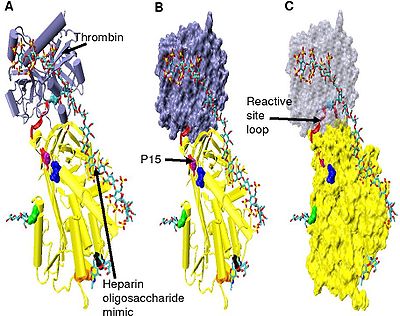 Increased thrombin inhibition requires the minimal heparin pentasaccharide plus at least an additional 13 monomeric units. This is thought to be due to a requirement that antithrombin and thrombin must bind to the same heparin chain adjacent to each other. This can be seen in the series of models shown in Figure 5.
Increased thrombin inhibition requires the minimal heparin pentasaccharide plus at least an additional 13 monomeric units. This is thought to be due to a requirement that antithrombin and thrombin must bind to the same heparin chain adjacent to each other. This can be seen in the series of models shown in Figure 5.
In the structures shown in Figure 5 the C-terminal portion (P' side) of the reactive site loop is in an extended conformation when compared with other un-activated or heparin activated antithrombin structures. The P' region of antithrombin is unusually long relative to the P' region of other serpins and in un-activated or heparin activated antithrombin structures forms a tightly hydrogen bond
ed β-turn. P' elongation occurs through the breaking of all hydrogen bonds involved in the β-turn.
The hinge region of antithrombin in the Figure 5 complex could not be modelled due to its conformational flexibility, and amino acids P9-P14 are not seen in this structure. This conformational flexibility indicates an equilibrium
may exist within the complex between a P14 P15 reactive site loop inserted antithrombin conformation and a P14 P15 reactive site loop expelled conformation. In support of this, analysis of the positioning of P15 Gly in the Figure 5 complex (labelled in model B) shows it to be inserted into beta sheet
A (see model C).
between the two is threefold for the pentasaccharide shown in Figure 3 and greater than tenfold for full length heparin, with β-antithrombin having a higher affinity. The higher affinity of β-antithrombin is thought to be due to the increased rate at which subsequent conformational changes occur within the protein upon initial heparin binding. For α-antithrombin, the additional glycosylation at Asn-135 is not thought to interfere with initial heparin binding, but rather to inhibit any resulting conformational changes.
Even though it is present at only 5–10% the levels of α-antithrombin, due to its increased heparin affinity, it is thought that β-antithrombin is more important than α-antithrombin in controlling thrombogenic events resulting from tissue injury. Indeed, thrombin inhibition after injury to the aorta
has been attributed solely to β-antithrombin.
antithrombin deficiencies and an increased risk of any affected individual developing thrombotic disease. Antithrombin deficiency generally comes to light when a patient suffers recurrent venous thrombosis
and pulmonary embolism
.
, premature birth
, kidney disease with protein loss in the urine in patients with nephrotic syndrome
,
or as a result of interventions such as major surgery
or cardiopulmonary bypass.
antithrombin analyses. Maintenance of an adequate level of antithrombin activity, which is at least 70% that of a normal functional level is essential to ensure effective inhibition of blood coagulation proteases. Typically as a result of type I or type II antithrombin deficiency, functional antithrombin levels are reduced to below 50% of normal.
Most cases of type I deficiency are due to point mutation
s, deletions or minor insertions within the antithrombin gene. These genetic mutations result in type I deficiency through a variety of mechanisms:
In the revised system of classification again adopted by the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis, type II antithrombin deficiency remains subdivided into three subgroups: the already mentioned type II PE, along with type II RS, were mutations effect the reactive site and type II HBS, were mutations effect the antithrombin heparin binding site. For the purposes of an antithrombin mutational database compiled by members of the Plasma Coagulation Inhibitors Subcommittee of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis, type IIa cases are now classified as type II PE, type IIb cases as type II RS and type IIc cases as type II HBS.
disorders.
It can made from the milk of goat
s that have been genetically modified
to produce it, such as the recently FDA-approved ATryn
.
 Cleavage at the reactive site results in entrapment of the thrombin protease, with movement of the cleaved reactive site loop together with the bound protease, such that the
Cleavage at the reactive site results in entrapment of the thrombin protease, with movement of the cleaved reactive site loop together with the bound protease, such that the
loop forms an extra sixth strand in the middle of beta sheet
A. This movement of the reactive site loop can also be induced without cleavage, with the resulting crystallographic structure being identical to that of the physiologically latent conformation of plasminogen activator inhibitor-1
(PAI-1). For this reason the conformation of antithrombin in which the reactive site loop is incorporated uncleaved into the main body of the protein is referred to as latent antithrombin. In contrast to PAI-1 the transition for antithrombin from a normal or native conformation to a latent conformation is irreversible.
Native antithrombin can be converted to latent antithrombin (L-antithrombin) by heating alone or heating in the presence of citrate
. However, without extreme heating and at 37°C (body temperature) 10% of all antithrombin circulating in the blood is converted to the L-antithrombin over a 24 hour period. The structure of L-antithrombin is shown in Figure 6.
The 3-dimensional structure of native antithrombin was first determined in 1994. Unexpectedly the protein crystallized as a heterodimer
composed of one molecule of native antithrombin and one molecule of latent antithrombin. Latent antithrombin on formation immediately links to a molecule of native antithrombin to form the heterodimer, and it is not until the concentration of latent antithrombin exceeds 50% of the total antithrombin that it can be detected analytically. Not only is the latent form of antithrombin inactive against its target coagulation proteases, but its dimerisation with an otherwise active native antithrombin molecule also results in the native molecules inactivation. The physiological impact of the loss of antithrombin activity either through latent antithrombin formation or through subsequent dimer formation is exacerbated by the preference for dimerisation to occur between heparin activated β-antithrombin and latent antithrombin as opposed to α-antithrombin.
A form of antithrombin that is an intermediate in the conversion between native and latent forms of antithrombin has also been isolated and this has been termed prelatent antithrombin.
is a physiological process involving the growth of new blood vessel
s from pre-existing vessels. Under normal physiological conditions angiogenesis is tightly regulated and is controlled by a balance of angiogenic stimulators and angiogenic inhibitors
. Tumor
growth is dependent upon angiogenesis and during tumor development a sustained production of angiogenic stimulatory factors is required along with a reduction in the quantity of angiogenic inhibitory factors tumor cells produce. The cleaved and latent form of antithrombin potently inhibit angiogenesis and tumor growth in animal models. The prelatent form of antithrombin has been shown to inhibit angiogenesis in-vitro but to date has not been tested in experimental animal models.
Coagulation
Coagulation is a complex process by which blood forms clots. It is an important part of hemostasis, the cessation of blood loss from a damaged vessel, wherein a damaged blood vessel wall is covered by a platelet and fibrin-containing clot to stop bleeding and begin repair of the damaged vessel...
system. Antithrombin is a glycoprotein
Glycoprotein
Glycoproteins are proteins that contain oligosaccharide chains covalently attached to polypeptide side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known as glycosylation. In proteins that have segments extending...
produced by the liver
Liver
The liver is a vital organ present in vertebrates and some other animals. It has a wide range of functions, including detoxification, protein synthesis, and production of biochemicals necessary for digestion...
and consists of 432 amino acids. It contains three disulfide bond
Disulfide bond
In chemistry, a disulfide bond is a covalent bond, usually derived by the coupling of two thiol groups. The linkage is also called an SS-bond or disulfide bridge. The overall connectivity is therefore R-S-S-R. The terminology is widely used in biochemistry...
s and a total of four possible glycosylation
Glycosylation
Glycosylation is the reaction in which a carbohydrate, i.e. a glycosyl donor, is attached to a hydroxyl or other functional group of another molecule . In biology glycosylation refers to the enzymatic process that attaches glycans to proteins, lipids, or other organic molecules...
sites. α-Antithrombin is the dominant form of antithrombin found in blood plasma
Blood plasma
Blood plasma is the straw-colored liquid component of blood in which the blood cells in whole blood are normally suspended. It makes up about 55% of the total blood volume. It is the intravascular fluid part of extracellular fluid...
and has an oligosaccharide occupying each of its four glycosylation sites. A single glycosylation site remains consistently un-occupied in the minor form of antithrombin, β-antithrombin. Its activity is increased manifold by the anticoagulant
Anticoagulant
An anticoagulant is a substance that prevents coagulation of blood. A group of pharmaceuticals called anticoagulants can be used in vivo as a medication for thrombotic disorders. Some anticoagulants are used in medical equipment, such as test tubes, blood transfusion bags, and renal dialysis...
drug heparin
Heparin
Heparin , also known as unfractionated heparin, a highly sulfated glycosaminoglycan, is widely used as an injectable anticoagulant, and has the highest negative charge density of any known biological molecule...
, which enhances the binding of antithrombin to factor II
Thrombin
Thrombin is a "trypsin-like" serine protease protein that in humans is encoded by the F2 gene. Prothrombin is proteolytically cleaved to form thrombin in the first step of the coagulation cascade, which ultimately results in the stemming of blood loss...
and factor X
Factor X
Factor X, also known by the eponym Stuart-Prower factor or as prothrombinase, is an enzyme of the coagulation cascade. It is a serine endopeptidase .-Physiology:...
.
Antithrombin nomenclature
Antithrombin is also termed Antithrombin III (AT III). The designations Antithrombin I through to Antithrombin IV originate in early studies carried out in the 1950s by Seegers, Johnson and Fell.Antithrombin I (AT I) refers to the absorption of thrombin
Thrombin
Thrombin is a "trypsin-like" serine protease protein that in humans is encoded by the F2 gene. Prothrombin is proteolytically cleaved to form thrombin in the first step of the coagulation cascade, which ultimately results in the stemming of blood loss...
onto fibrin
Fibrin
Fibrin is a fibrous, non-globular protein involved in the clotting of blood. It is a fibrillar protein that is polymerised to form a "mesh" that forms a hemostatic plug or clot over a wound site....
after thrombin has activated fibrinogen
Fibrinogen
Fibrinogen is a soluble plasma glycoprotein, synthesised by the liver, that is converted by thrombin into fibrin during blood coagulation. This is achieved through processes in the coagulation cascade that activate the zymogen prothrombin to the serine protease thrombin, which is responsible for...
. Antithrombin II (AT II) refers to a cofactor in plasma, which together with heparin
Heparin
Heparin , also known as unfractionated heparin, a highly sulfated glycosaminoglycan, is widely used as an injectable anticoagulant, and has the highest negative charge density of any known biological molecule...
interferes with the interaction of thrombin
Thrombin
Thrombin is a "trypsin-like" serine protease protein that in humans is encoded by the F2 gene. Prothrombin is proteolytically cleaved to form thrombin in the first step of the coagulation cascade, which ultimately results in the stemming of blood loss...
and fibrinogen
Fibrinogen
Fibrinogen is a soluble plasma glycoprotein, synthesised by the liver, that is converted by thrombin into fibrin during blood coagulation. This is achieved through processes in the coagulation cascade that activate the zymogen prothrombin to the serine protease thrombin, which is responsible for...
. Antithrombin III (AT III) refers to a substance in plasma
Blood plasma
Blood plasma is the straw-colored liquid component of blood in which the blood cells in whole blood are normally suspended. It makes up about 55% of the total blood volume. It is the intravascular fluid part of extracellular fluid...
that inactivates thrombin. Antithrombin IV (AT IV) refers to an antithrombin that becomes activated during and shortly after blood coagulation. Only AT III and possibly AT I are medically significant. AT III is generally referred to solely as "Antithrombin" and it is Antithrombin III that is discussed in this article.
Structure

Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
in blood plasma
Blood plasma
Blood plasma is the straw-colored liquid component of blood in which the blood cells in whole blood are normally suspended. It makes up about 55% of the total blood volume. It is the intravascular fluid part of extracellular fluid...
of around 3 days.
The normal antithrombin concentration in human blood plasma
Blood plasma
Blood plasma is the straw-colored liquid component of blood in which the blood cells in whole blood are normally suspended. It makes up about 55% of the total blood volume. It is the intravascular fluid part of extracellular fluid...
is high at approximately 0.12 mg/ml, which is equivalent to a molar concentration of 2.3 μM.
Antithrombin has been isolated from the plasma of a large number of species additional to humans. As deduced from protein and cDNA sequencing, cow, sheep, rabbit and mouse antithrombins are all 433 amino acids in length, which is one amino acid longer than human antithrombin. The extra amino acid is thought to occur at amino acid position 6. Cow, sheep, rabbit, mouse, and human antithrombins share between 84 and 89% amino acid sequence identity. Six of the amino acids form three intramolecular disulfide bond
Disulfide bond
In chemistry, a disulfide bond is a covalent bond, usually derived by the coupling of two thiol groups. The linkage is also called an SS-bond or disulfide bridge. The overall connectivity is therefore R-S-S-R. The terminology is widely used in biochemistry...
s, Cys
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
8-Cys128, Cys21-Cys95, and Cys248-Cys430.
They all have four potential N-glycosylation sites. These occur at asparagine
Asparagine
Asparagine is one of the 20 most common natural amino acids on Earth. It has carboxamide as the side-chain's functional group. It is not an essential amino acid...
(Asn) amino acid numbers 96, 135, 155, and 192 in humans and at similar amino acid numbers in other species. All these sites are occupied by covalently attached oligosaccharide side-chains in the predominant form of human antithrombin, α-antithrombin, resulting in a molecular weight for this form of antithrombin of 58,200. The potential glycosylation site at asparagine 135 is not occupied in a minor form (around 10%) of antithrombin, β-antithrombin (see Figure 1).
Recombinant
Recombinant DNA
Recombinant DNA molecules are DNA sequences that result from the use of laboratory methods to bring together genetic material from multiple sources, creating sequences that would not otherwise be found in biological organisms...
antithrombins with properties similar to those of normal human antithrombin have been produced using baculovirus
Baculovirus
The baculoviruses are a family of large rod-shaped viruses that can be divided to two genera: nucleopolyhedroviruses and granuloviruses . While GVs contain only one nucleocapsid per envelope, NPVs contain either single or multiple nucleocapsids per envelope. The enveloped virions are further...
-infected insect cells and mammalian cell lines grown in cell culture
Cell culture
Cell culture is the complex process by which cells are grown under controlled conditions. In practice, the term "cell culture" has come to refer to the culturing of cells derived from singlecellular eukaryotes, especially animal cells. However, there are also cultures of plants, fungi and microbes,...
. These recombinant antithrombins generally have different glycosylation patterns to normal antithrombin and are typically used in antithrombin structural studies. For this reason many of the antithrombin structures stored in the protein data bank
Protein Data Bank
The Protein Data Bank is a repository for the 3-D structural data of large biological molecules, such as proteins and nucleic acids....
and presented in this article show variable glycosylation patterns.
Antithrombin begins in its native state, which has a higher free energy compared to the latent state, which it decays to on average after 3 days. The latent state has the same form as the activated state - that is, when it is inhibiting thrombin. As such it is a classic example of the utility of kinetic vs thermodynamic control of protein folding.
Function

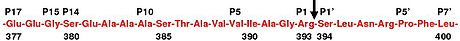
Blood plasma
Blood plasma is the straw-colored liquid component of blood in which the blood cells in whole blood are normally suspended. It makes up about 55% of the total blood volume. It is the intravascular fluid part of extracellular fluid...
protease
Protease
A protease is any enzyme that conducts proteolysis, that is, begins protein catabolism by hydrolysis of the peptide bonds that link amino acids together in the polypeptide chain forming the protein....
inhibitor
Enzyme inhibitor
An enzyme inhibitor is a molecule that binds to enzymes and decreases their activity. Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors. They are also used as herbicides and pesticides...
s, such as alpha 1-antichymotrypsin
Alpha 1-antichymotrypsin
Alpha 1-antichymotrypsin is an alpha globulin glycoprotein that is a member of the serpin superfamily.It inhibits the activity of certain enzymes called proteases, such as cathepsin G that is found in neutrophils, and chymases found in mast cells, by cleaving them into a different shape or...
, alpha 2-antiplasmin
Alpha 2-antiplasmin
Alpha 2-antiplasmin is a serine protease inhibitor responsible for inactivating plasmin, an important enzyme that participates in fibrinolysis and degradation of various other proteins...
and Heparin cofactor II
Heparin cofactor II
Heparin cofactor II, a protein encoded by the SERPIND1 gene, is a coagulation factor that inhibits IIa, and is a cofactor for heparin and dermatan sulfate...
.
The physiological target protease
Protease
A protease is any enzyme that conducts proteolysis, that is, begins protein catabolism by hydrolysis of the peptide bonds that link amino acids together in the polypeptide chain forming the protein....
s of antithrombin are those of the contact activation pathway (formerly known as the intrinsic pathway), namely the activated forms of Factor X
Factor X
Factor X, also known by the eponym Stuart-Prower factor or as prothrombinase, is an enzyme of the coagulation cascade. It is a serine endopeptidase .-Physiology:...
(Xa), Factor IX
Factor IX
Factor IX is one of the serine proteases of the coagulation system; it belongs to peptidase family S1. Deficiency of this protein causes hemophilia B. It was discovered in 1952 after a young boy named Stephen Christmas was found to be lacking this exact factor, leading to...
(IXa), Factor XI
Factor XI
Factor XI or plasma thromboplastin antecedent is the zymogen form of factor XIa, one of the enzymes of the coagulation cascade. Like many other coagulation factors, it is a serine protease. In humans, Factor XI is encoded by the F11 gene....
(XIa), Factor XII
Factor XII
Coagulation factor XII also known as Hageman factor is a plasma protein. It is the zymogen form of factor XIIa, an enzyme of the serine protease class. In humans, factor XII is encoded by the F12 gene.- Function :...
(XIIa) and, to a greater extent, Factor II (thrombin) (IIa), and also the activated form of Factor VII
Factor VII
Factor VII is one of the proteins that causes blood to clot in the coagulation cascade. It is an enzyme of the serine protease class. A recombinant form of human factor VIIa has U.S. Food and Drug Administration approval for uncontrolled bleeding in hemophilia patients...
(VIIa) from the tissue factor pathway (formerly known as the extrinsic pathway). The inhibitor also inactivates kallikrein
Kallikrein
Kallikreins are a subgroup of serine proteases, enzymes capable of cleaving peptide bonds in proteins. In humans, plasma kallikrein has no known homologue, while tissue kallikrein-related peptidases encode a family of fifteen closely related serine proteases...
and plasmin
Plasmin
Plasmin is an important enzyme present in blood that degrades many blood plasma proteins, most notably, fibrin clots. The degradation of fibrin is termed fibrinolysis. In humans, the plasmin protein is encoded by the PLG gene.- Function :...
, also involved in blood coagulation. However it inactivates certain other serine proteases that are not involved in coagulation such as trypsin
Trypsin
Trypsin is a serine protease found in the digestive system of many vertebrates, where it hydrolyses proteins. Trypsin is produced in the pancreas as the inactive proenzyme trypsinogen. Trypsin cleaves peptide chains mainly at the carboxyl side of the amino acids lysine or arginine, except when...
and the C1s subunit of the enzyme C1 involved in the classical complement pathway
Classical complement pathway
The Classical pathway of activation of the complement system is a group of blood proteins that mediate the specific antibody response. The main activators of the Classical Pathway are antigen-antibody complexes.-Initiation:...
.
Protease inactivation results as a consequence of trapping the protease in an equimolar complex with antithrombin in which the active site of the protease enzyme is inaccessible to its usual substrate
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
. The formation of an antithrombin-protease complex involves an interaction between the protease and a specific reactive peptide bond
Peptide bond
This article is about the peptide link found within biological molecules, such as proteins. A similar article for synthetic molecules is being created...
within antithrombin. In human antithrombin this bond is between arginine
Arginine
Arginine is an α-amino acid. The L-form is one of the 20 most common natural amino acids. At the level of molecular genetics, in the structure of the messenger ribonucleic acid mRNA, CGU, CGC, CGA, CGG, AGA, and AGG, are the triplets of nucleotide bases or codons that codify for arginine during...
(arg) 393 and serine
Serine
Serine is an amino acid with the formula HO2CCHCH2OH. It is one of the proteinogenic amino acids. By virtue of the hydroxyl group, serine is classified as a polar amino acid.-Occurrence and biosynthesis:...
(ser) 394 (see Figure 2 and Figure 3).
It is thought that protease enzymes become trapped in inactive antithrombin-protease complexes as a consequence of their attack on the reactive bond. Although attacking a similar bond within the normal protease substrate results in rapid proteolytic cleavage of the substrate, initiating an attack on the antithrombin reactive bond causes antithrombin to become activated and trap the enzyme at an intermediate stage of the proteolytic process. Given time, thrombin is able to cleave the reactive bond within antithrombin and an inactive antithrombin-thrombin complex will dissociate, however the time it takes for this to occur may be greater than 3 days. However bonds P3-P4 and P1'-P2' can be rapidly cleaved by neutrophil elastase and the bacterial enzyme thermolysin
Thermolysin
Thermolysin is a thermostable neutral metalloproteinase enzyme produced by the gram-positive bacteria Bacillus thermoproteolyticus. It requires one zinc ion for enzyme activity and four calcium ions for structural stability. Thermolysin specifically catalyzes the hydrolysis of peptide bonds...
respectively, resulting in inactive antithrombins no longer able to inhibit thrombin activity.
The rate of antithrombin's inhibition of protease activity is greatly enhanced by its additional binding to heparin
Heparin
Heparin , also known as unfractionated heparin, a highly sulfated glycosaminoglycan, is widely used as an injectable anticoagulant, and has the highest negative charge density of any known biological molecule...
as is its inactivation by neutrophil elastase.
Antithrombin and heparin
Antithrombin inactivates its physiological target enzymes, Thrombin, Factor Xa and Factor IXa with rate constants of 7–11 x 103, 2.5 x 103 M−1 s−1 and 1 x 10 M−1 s−1 respectively. The rate of antithrombin-thrombin inactivation increases to 1.5 - 4 x 107 M−1 s−1 in the presence of heparin, i.e. the reaction is accelerated 2000-4000 fold. Factor Xa inhibition is accelerated by only 500 to 1000 fold in the presence of heparin and the maximal rate constant is 10 fold lower than that of thrombin inhibition. The rate enhancement of antithrombin-Factor IXa inhibition shows an approximate 1 million fold enhancement in the presence of heparin and physiological levels of calciumCalcium
Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust...
.
AT-III binds to a specific pentasaccharide sulfation sequence contained within the heparin polymer
GlcNAc/NS(6S)-GlcA-GlcNS(3S,6S)-IdoA(2S)-GlcNS(6S)
Upon binding to this pentasaccharide sequence, inhibition of protease activity is increased by heparin as a result of two distinct mechanisms. In one mechanism heparin stimulation of Factor IXa and Xa inhibition depends on a conformational change within antithrombin involving the reactive site loop and is thus allosteric. In another mechanism stimulation of thrombin inhibition depends on the formation of a ternary complex
Ternary complex
A Ternary complex refers to a protein complex containing three different molecules which are bound together. In structural biology ternary complex can be used to describe a crystal containing a protein with two small molecules bound, for example cofactor and substrate; or a complex formed between...
between AT-III, thrombin, and heparin.
Allosteric activation

In the absence of heparin, amino acids P14 and P15 (see Figure 3) from the reactive site loop are embedded within the main body of the protein (specifically the top of beta sheet
Beta sheet
The β sheet is the second form of regular secondary structure in proteins, only somewhat less common than the alpha helix. Beta sheets consist of beta strands connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet...
A). This feature is in common with other serpin
Serpin
Serpins are a group of proteins with similar structures that were first identified as a set of proteins able to inhibit proteases. The acronym serpin was originally coined because many serpins inhibit chymotrypsin-like serine proteases .The first members of the serpin superfamily to be extensively...
s such as heparin cofactor II
Heparin cofactor II
Heparin cofactor II, a protein encoded by the SERPIND1 gene, is a coagulation factor that inhibits IIa, and is a cofactor for heparin and dermatan sulfate...
, alpha 1-antichymotrypsin
Alpha 1-antichymotrypsin
Alpha 1-antichymotrypsin is an alpha globulin glycoprotein that is a member of the serpin superfamily.It inhibits the activity of certain enzymes called proteases, such as cathepsin G that is found in neutrophils, and chymases found in mast cells, by cleaving them into a different shape or...
and MENT
Myeloid and erythroid nuclear termination stage specific protein
Myeloid and erythroid nuclear termination stage-specific protein is a member of the serpin family of protease inhibitors, and participates in DNA and chromatin condensation. Alongside its ability to condense chromatin, MENT is also an effective inhibitor of the proteases cathepsin K, cathepsin L,...
.
The conformational change most relevant for Factor IXa and Xa inhibition involves the P14 and P15 amino acids within the N-terminal region of the reactive site loop (circled in Figure 4 model B). This region has been termed the hinge region. The conformational change within the hinge region in response to heparin binding results in the expulsion of P14 and P15 from the main body of the protein and it has been shown that by preventing this conformational change, increased Factor IXa and Xa inhibition does not occur. It is thought that the increased flexibility given to the reactive site loop as a result of the hinge region conformational change is a key factor in influencing increased Factor IXa and Xa inhibition. It has been calculated that in the absence of the pentasaccharide only one in every 400 Antithrombin molecules (0.25%) is in an active conformation with the P14 and P15 amino acids expelled.
Non-allosteric activation

In the structures shown in Figure 5 the C-terminal portion (P' side) of the reactive site loop is in an extended conformation when compared with other un-activated or heparin activated antithrombin structures. The P' region of antithrombin is unusually long relative to the P' region of other serpins and in un-activated or heparin activated antithrombin structures forms a tightly hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
ed β-turn. P' elongation occurs through the breaking of all hydrogen bonds involved in the β-turn.
The hinge region of antithrombin in the Figure 5 complex could not be modelled due to its conformational flexibility, and amino acids P9-P14 are not seen in this structure. This conformational flexibility indicates an equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
may exist within the complex between a P14 P15 reactive site loop inserted antithrombin conformation and a P14 P15 reactive site loop expelled conformation. In support of this, analysis of the positioning of P15 Gly in the Figure 5 complex (labelled in model B) shows it to be inserted into beta sheet
Beta sheet
The β sheet is the second form of regular secondary structure in proteins, only somewhat less common than the alpha helix. Beta sheets consist of beta strands connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet...
A (see model C).
Effect of glycosylation on activity
α-Antithrombin and β-antithrombin differ in their affinity for heparin. The difference in dissociation constantDissociation constant
In chemistry, biochemistry, and pharmacology, a dissociation constant is a specific type of equilibrium constant that measures the propensity of a larger object to separate reversibly into smaller components, as when a complex falls apart into its component molecules, or when a salt splits up into...
between the two is threefold for the pentasaccharide shown in Figure 3 and greater than tenfold for full length heparin, with β-antithrombin having a higher affinity. The higher affinity of β-antithrombin is thought to be due to the increased rate at which subsequent conformational changes occur within the protein upon initial heparin binding. For α-antithrombin, the additional glycosylation at Asn-135 is not thought to interfere with initial heparin binding, but rather to inhibit any resulting conformational changes.
Even though it is present at only 5–10% the levels of α-antithrombin, due to its increased heparin affinity, it is thought that β-antithrombin is more important than α-antithrombin in controlling thrombogenic events resulting from tissue injury. Indeed, thrombin inhibition after injury to the aorta
Aorta
The aorta is the largest artery in the body, originating from the left ventricle of the heart and extending down to the abdomen, where it branches off into two smaller arteries...
has been attributed solely to β-antithrombin.
Role in disease
Evidence for the important role antithrombin plays in regulating normal blood coagulation is demonstrated by the correlation between inherited or acquiredAcquired disorder
An acquired disorder is a medical condition which develops post-fetally; in contrast with a congenital disorder, which is present at birth. A congenital disorder may be antecedent to an acquired disorder ....
antithrombin deficiencies and an increased risk of any affected individual developing thrombotic disease. Antithrombin deficiency generally comes to light when a patient suffers recurrent venous thrombosis
Thrombosis
Thrombosis is the formation of a blood clot inside a blood vessel, obstructing the flow of blood through the circulatory system. When a blood vessel is injured, the body uses platelets and fibrin to form a blood clot to prevent blood loss...
and pulmonary embolism
Pulmonary embolism
Pulmonary embolism is a blockage of the main artery of the lung or one of its branches by a substance that has travelled from elsewhere in the body through the bloodstream . Usually this is due to embolism of a thrombus from the deep veins in the legs, a process termed venous thromboembolism...
.
Acquired antithrombin deficiency
Acquired antithrombin deficiency may result from a range of disorders such as liver dysfunction (coagulopathy), sepsisSepsis
Sepsis is a potentially deadly medical condition that is characterized by a whole-body inflammatory state and the presence of a known or suspected infection. The body may develop this inflammatory response by the immune system to microbes in the blood, urine, lungs, skin, or other tissues...
, premature birth
Premature birth
In humans preterm birth refers to the birth of a baby of less than 37 weeks gestational age. The cause for preterm birth is in many situations elusive and unknown; many factors appear to be associated with the development of preterm birth, making the reduction of preterm birth a challenging...
, kidney disease with protein loss in the urine in patients with nephrotic syndrome
Nephrotic syndrome
Nephrotic syndrome is a nonspecific disorder in which the kidneys are damaged, causing them to leak large amounts of protein from the blood into the urine....
,
or as a result of interventions such as major surgery
Surgery
Surgery is an ancient medical specialty that uses operative manual and instrumental techniques on a patient to investigate and/or treat a pathological condition such as disease or injury, or to help improve bodily function or appearance.An act of performing surgery may be called a surgical...
or cardiopulmonary bypass.
Inherited antithrombin deficiency
The incidence of inherited antithrombin deficiency has been estimated at between 1:2000 and 1:5000 in the normal population, with the first family suffering from inherited antithrombin deficiency being described in 1965. Subsequently it was proposed that the classification of inherited antithrombin deficiency be designated as either type I or type II, based upon functional and immunochemicalImmunoelectrophoresis
Immunoelectrophoresis is a general name for a number of biochemical methods for separation and characterization of proteins based on electrophoresis and reaction with antibodies. All variants of immunoelectrophoresis require immunoglobulins, also known as antibodies reacting with the proteins to be...
antithrombin analyses. Maintenance of an adequate level of antithrombin activity, which is at least 70% that of a normal functional level is essential to ensure effective inhibition of blood coagulation proteases. Typically as a result of type I or type II antithrombin deficiency, functional antithrombin levels are reduced to below 50% of normal.
Type I antithrombin deficiency
Type I antithrombin deficiency is characterised by a decrease in both antithrombin activity and antithrombin concentration in the blood of affected individuals. Type I deficiency was originally further divided into two subgroups, Ia and Ib, based upon heparin affinity. The antithrombin of subgroup Ia individuals showed a normal affinity for heparin while the antithrombin of subgroup Ib individuals showed a reduced affinity for heparin. Subsequent functional analysis of a group of 1b cases found them not only to have reduced heparin affinity but multiple or 'pleiotrophic' abnormalities affecting the reactive site, the heparin binding site and antithrombin blood concentration. In a revised system of classification adopted by the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis, type Ib cases are now designated as type II PE, Pleiotrophic effect.Most cases of type I deficiency are due to point mutation
Point mutation
A point mutation, or single base substitution, is a type of mutation that causes the replacement of a single base nucleotide with another nucleotide of the genetic material, DNA or RNA. Often the term point mutation also includes insertions or deletions of a single base pair...
s, deletions or minor insertions within the antithrombin gene. These genetic mutations result in type I deficiency through a variety of mechanisms:
- Mutations may produce unstable antithrombins that either may be not exported into the blood correctly upon completion biosynthesis or exist in the blood for a shortened period of time, e.g., the deletion of 6 base pairBase pairIn molecular biology and genetics, the linking between two nitrogenous bases on opposite complementary DNA or certain types of RNA strands that are connected via hydrogen bonds is called a base pair...
s in codons 106-108. - Mutations may affect mRNA processing of the antithrombin gene.
- Minor insertions or deletions may lead to frame shift mutations and premature termination of the antithrombin gene.
- Point mutations may also result in the premature generation of a termination or stop codonStop codonIn the genetic code, a stop codon is a nucleotide triplet within messenger RNA that signals a termination of translation. Proteins are based on polypeptides, which are unique sequences of amino acids. Most codons in messenger RNA correspond to the addition of an amino acid to a growing polypeptide...
e.g. the mutation of codon 129, CGA→TGA (UGA after transcription), replaces a normal codon for arginine with a termination codon.
Type II antithrombin deficiency
Type II antithrombin deficiency is characterised by normal antithrombin levels but reduced antithrombin activity in the blood of affected individuals. Originally it was proposed that type II deficiency be further divided into three subgroups IIa, IIb and IIc depending upon which antithrombin functional activity is reduced or retained.- Subgroup IIa - Decreased thrombin inactivation, decreased factor Xa inactivation and decreased heparin affinity.
- Subgroup IIb - Decreased thrombin inactivation and normal heparin affinity.
- Subgroup IIc - Normal thrombin inactivation, normal factor Xa inactivation and decreased heparin affinity.
In the revised system of classification again adopted by the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis, type II antithrombin deficiency remains subdivided into three subgroups: the already mentioned type II PE, along with type II RS, were mutations effect the reactive site and type II HBS, were mutations effect the antithrombin heparin binding site. For the purposes of an antithrombin mutational database compiled by members of the Plasma Coagulation Inhibitors Subcommittee of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis, type IIa cases are now classified as type II PE, type IIb cases as type II RS and type IIc cases as type II HBS.
Toponyms
Presently it is relatively easy to characterise a specific antithrombin genetic mutation. However prior to the use of modern characterisation techniques investigators named mutations for the town or city were the individual suffering from the deficiency resided i.e. the antithrombin mutation was designated a toponym. Modern mutational characterisation has since shown that many individual antithrombin toponyms are actually the result of the same genetic mutation, for example Antithrombin-Toyama, is equivalent to Antihrombin-Kumamoto, -Amien, -Tours, -Paris-1, -Paris-2, -Alger, -Padua-2 and -Barcelona.As medication
Antithrombin may be given exogenously as a medication for thromboticThrombosis
Thrombosis is the formation of a blood clot inside a blood vessel, obstructing the flow of blood through the circulatory system. When a blood vessel is injured, the body uses platelets and fibrin to form a blood clot to prevent blood loss...
disorders.
It can made from the milk of goat
Goat
The domestic goat is a subspecies of goat domesticated from the wild goat of southwest Asia and Eastern Europe. The goat is a member of the Bovidae family and is closely related to the sheep as both are in the goat-antelope subfamily Caprinae. There are over three hundred distinct breeds of...
s that have been genetically modified
Genetic engineering
Genetic engineering, also called genetic modification, is the direct human manipulation of an organism's genome using modern DNA technology. It involves the introduction of foreign DNA or synthetic genes into the organism of interest...
to produce it, such as the recently FDA-approved ATryn
ATryn
ATryn is the brand name of the anticoagulant antithrombin manufactured by the Massachusetts-based U.S. company GTC Biotherapeutics. It is made from the milk of goats that have been genetically modified to produce human antithrombin, a plasma protein with anticoagulant properties. Microinjection was...
.
Cleaved and latent antithrombin

loop forms an extra sixth strand in the middle of beta sheet
Beta sheet
The β sheet is the second form of regular secondary structure in proteins, only somewhat less common than the alpha helix. Beta sheets consist of beta strands connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet...
A. This movement of the reactive site loop can also be induced without cleavage, with the resulting crystallographic structure being identical to that of the physiologically latent conformation of plasminogen activator inhibitor-1
Plasminogen activator inhibitor-1
Plasminogen activator inhibitor-1 also known as endothelial plasminogen activator inhibitor or serpin E1 is a protein that in humans is encoded by the SERPINE1 gene....
(PAI-1). For this reason the conformation of antithrombin in which the reactive site loop is incorporated uncleaved into the main body of the protein is referred to as latent antithrombin. In contrast to PAI-1 the transition for antithrombin from a normal or native conformation to a latent conformation is irreversible.
Native antithrombin can be converted to latent antithrombin (L-antithrombin) by heating alone or heating in the presence of citrate
Citrate
A citrate can refer either to the conjugate base of citric acid, , or to the esters of citric acid. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate.-Other citric acid ions:...
. However, without extreme heating and at 37°C (body temperature) 10% of all antithrombin circulating in the blood is converted to the L-antithrombin over a 24 hour period. The structure of L-antithrombin is shown in Figure 6.
The 3-dimensional structure of native antithrombin was first determined in 1994. Unexpectedly the protein crystallized as a heterodimer
Protein dimer
In biochemistry, a dimer is a macromolecular complex formed by two, usually non-covalently bound, macromolecules like proteins or nucleic acids...
composed of one molecule of native antithrombin and one molecule of latent antithrombin. Latent antithrombin on formation immediately links to a molecule of native antithrombin to form the heterodimer, and it is not until the concentration of latent antithrombin exceeds 50% of the total antithrombin that it can be detected analytically. Not only is the latent form of antithrombin inactive against its target coagulation proteases, but its dimerisation with an otherwise active native antithrombin molecule also results in the native molecules inactivation. The physiological impact of the loss of antithrombin activity either through latent antithrombin formation or through subsequent dimer formation is exacerbated by the preference for dimerisation to occur between heparin activated β-antithrombin and latent antithrombin as opposed to α-antithrombin.
A form of antithrombin that is an intermediate in the conversion between native and latent forms of antithrombin has also been isolated and this has been termed prelatent antithrombin.
Antiangiogenic antithrombin
AngiogenesisAngiogenesis
Angiogenesis is the physiological process involving the growth of new blood vessels from pre-existing vessels. Though there has been some debate over terminology, vasculogenesis is the term used for spontaneous blood-vessel formation, and intussusception is the term for the formation of new blood...
is a physiological process involving the growth of new blood vessel
Blood vessel
The blood vessels are the part of the circulatory system that transports blood throughout the body. There are three major types of blood vessels: the arteries, which carry the blood away from the heart; the capillaries, which enable the actual exchange of water and chemicals between the blood and...
s from pre-existing vessels. Under normal physiological conditions angiogenesis is tightly regulated and is controlled by a balance of angiogenic stimulators and angiogenic inhibitors
Angiogenesis inhibitor
An angiogenesis inhibitor is a substance that inhibits the growth of new blood vessels . Some angiogenesis inhibitors are a normal part of the body's control, some are administered as drugs, and some come from diet....
. Tumor
Tumor
A tumor or tumour is commonly used as a synonym for a neoplasm that appears enlarged in size. Tumor is not synonymous with cancer...
growth is dependent upon angiogenesis and during tumor development a sustained production of angiogenic stimulatory factors is required along with a reduction in the quantity of angiogenic inhibitory factors tumor cells produce. The cleaved and latent form of antithrombin potently inhibit angiogenesis and tumor growth in animal models. The prelatent form of antithrombin has been shown to inhibit angiogenesis in-vitro but to date has not been tested in experimental animal models.
External links
- The MEROPSMeropsMerops may refer to:* Merops , a genus of bee-eaters.* MEROPS, an on-line database for peptidases.It may also refer to several figures from Greek mythology:* King of Ethiopia, husband of Clymene, who lay with Helios and bore Phaethon...
online database for peptidases and their inhibitors: I04.018

