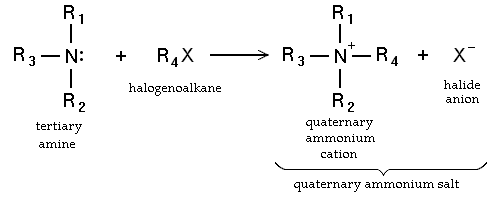Amine alkylation
Encyclopedia
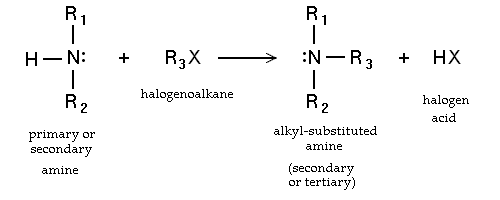
Amine alkylation is a type of organic reaction
between an alkyl halide and ammonia
or an amine
. The reaction is called nucleophilic aliphatic substitution (of the halide), and the reaction product is a higher substituted amine. The method is widely used in the laboratory, but is less important industrially, where alkyl halides are not preferred alkylating agents.
When the amine is a tertiary amine the reaction product is a quaternary ammonium salt in the Menshutkin reaction
:
Amines and ammonia are generally sufficiently basic to undergo direct alkylation, often under mild conditions. The reactions are difficult to control because the reaction product (a primary amine or a secondary amine) are often more nucleophilic than the precursor and will thus preferentially react with the alkylating agent. For example, reaction of 1-bromooctane with ammonia yields almost equal amounts of the primary amine and the secondary amine. Therefore, for laboratory purposes, N-alkylation is often limited to the synthesis of tertiary amines. A notable exception is the reactivity of alpha-halo carboxylic acids that do permit synthesis of primary amines with ammonia. Intramolecular
reactions of haloamines X-(CH2)n-NH2 give cyclic aziridine
s, azetidine
s and pyrrolidine
s.
N-alkylation is a general and useful route to quaternary ammonium salts from tertiary amines, because overalkylation is not possible.
Examples of N-alkylation with alkyl halides are the syntheses of benzylaniline, 1-benzylindole, and azetidine
. Another example is found in the derivatization of cyclen
. Industrially, ethylenediamine is produced by alkylation of ammonia with 1,2-dichloroethane
.
or para
to the halogen atom. For the arylation of amines with unactivated aryl halides, the Buchwald-Hartwig reaction
is useful. In this process, palladium complexes serve as catalysts.
, resulting in approximately 500,000 tons/y of methylamine
, dimethylamine
, and trimethylamine
. The reaction is poorly selective, requiring separation of the three products. Many other industrially significant alkyl amines are produced, again on a large scale, from the alcohols. Epoxide
s are another class of halide-free N-alkylating agents, useful in the production of ethanolamine
s.
, which uses hexamine
. The Gabriel synthesis
, involving the use of an equivalent to NH2-, only applies to primary alkyl halides.
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
between an alkyl halide and ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
or an amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
. The reaction is called nucleophilic aliphatic substitution (of the halide), and the reaction product is a higher substituted amine. The method is widely used in the laboratory, but is less important industrially, where alkyl halides are not preferred alkylating agents.
When the amine is a tertiary amine the reaction product is a quaternary ammonium salt in the Menshutkin reaction
Menshutkin reaction
The Menshutkin reaction in organic chemistry converts a tertiary amine to a quaternary ammonium salt by reaction with an alkyl halide:The reaction has been named after its discoverer, the Russian chemist Nikolai Menshutkin, who described the procedure in 1890...
:
Amines and ammonia are generally sufficiently basic to undergo direct alkylation, often under mild conditions. The reactions are difficult to control because the reaction product (a primary amine or a secondary amine) are often more nucleophilic than the precursor and will thus preferentially react with the alkylating agent. For example, reaction of 1-bromooctane with ammonia yields almost equal amounts of the primary amine and the secondary amine. Therefore, for laboratory purposes, N-alkylation is often limited to the synthesis of tertiary amines. A notable exception is the reactivity of alpha-halo carboxylic acids that do permit synthesis of primary amines with ammonia. Intramolecular
Intramolecular
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.- Examples :...
reactions of haloamines X-(CH2)n-NH2 give cyclic aziridine
Aziridine
Aziridines are organic compounds containing the aziridine functional group, a three-membered heterocycle with one amine group and two methylene groups...
s, azetidine
Azetidine
Azetidine is a heterocyclic organic compound. It belongs to the class of four membered rings and it contains a nitrogen atom.-External links:* *...
s and pyrrolidine
Pyrrolidine
Pyrrolidine, also known as tetrahydropyrrole, is an organic compound with the molecular formula C4H9N. It is a cyclic secondary amine with a five-membered heterocycle containing four carbon atoms and one nitrogen atom...
s.
N-alkylation is a general and useful route to quaternary ammonium salts from tertiary amines, because overalkylation is not possible.
Examples of N-alkylation with alkyl halides are the syntheses of benzylaniline, 1-benzylindole, and azetidine
Azetidine
Azetidine is a heterocyclic organic compound. It belongs to the class of four membered rings and it contains a nitrogen atom.-External links:* *...
. Another example is found in the derivatization of cyclen
Cyclen
Cyclen or 1,4,7,10-tetraazacyclododecane is a macrocycle and the aza analogue of the crown ether 12-crown-4. Derivatives of cyclen are larger cyclic polyamines but the repeating unit is always the same. Like crown ethers, cyclen compounds are capable of selectively binding cations...
. Industrially, ethylenediamine is produced by alkylation of ammonia with 1,2-dichloroethane
1,2-Dichloroethane
The chemical compound 1,2-dichloroethane, commonly known by its old name of ethylene dichloride , is a chlorinated hydrocarbon, mainly used to produce vinyl chloride monomer , the major precursor for PVC production. It is a colourless liquid with a chloroform-like odour...
.
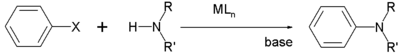
Aniline and related aryl derivatives
Traditionally, aryl halides (ArX) alkylate amines only reluctantly. The reaction usually requires "activated" aryl halides, such as those with strong electron-withdrawing groups such as nitro groups orthoArene substitution patterns
Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon.- Ortho, meta, and para substitution :...
or para
Arene substitution patterns
Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon.- Ortho, meta, and para substitution :...
to the halogen atom. For the arylation of amines with unactivated aryl halides, the Buchwald-Hartwig reaction
Buchwald-Hartwig reaction
The Buchwald–Hartwig reaction is a chemical reaction used in organic chemistry for the synthesis of carbon–nitrogen bonds via the palladium-catalyzed cross-coupling of amines with aryl halides. Though publications with similar focus were published as early as 1983, credit for its development is...
is useful. In this process, palladium complexes serve as catalysts.
Alkylation using alcohols
Industrially, most alkylations are typically conducted using alcohols, not alkyl halides. Alcohols are less expensive than alkyl halides and their alkylation does not produce salts, the disposal of which is be problematic. Key to the alkylation of alcohols is the use of catalysts that render the hydroxyl group a good leaving group. The largest scale N-alkylation is the production of the methylamines from ammonia and methanolMethanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
, resulting in approximately 500,000 tons/y of methylamine
Methylamine
Methylamine is the organic compound with a formula of CH3NH2. This colourless gas is a derivative of ammonia, but with one H atom replaced by a methyl group. It is the simplest primary amine. It is sold as a solution in methanol, ethanol, THF, and water, or as the anhydrous gas in pressurized...
, dimethylamine
Dimethylamine
Dimethylamine is an organic compound with the formula 2NH. This secondary amine is a colorless, flammable liquified gas with an ammonia-like odor. Dimethylamine is generally encountered as a solution in water at concentrations up to around 40%...
, and trimethylamine
Trimethylamine
Trimethylamine is an organic compound with the formula N3. This colorless, hygroscopic, and flammable tertiary amine has a strong "fishy" odor in low concentrations and an ammonia-like odor at higher concentrations...
. The reaction is poorly selective, requiring separation of the three products. Many other industrially significant alkyl amines are produced, again on a large scale, from the alcohols. Epoxide
Epoxide
An epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in...
s are another class of halide-free N-alkylating agents, useful in the production of ethanolamine
Ethanolamine
Ethanolamine, also called 2-aminoethanol or monoethanolamine , is an organic chemical compound that is both a primary amine and a primary alcohol . Like other amines, monoethanolamine acts as a weak base...
s.
Alternative alkylation methods
For laboratory use, the N-alkylation reaction is often unselective. A variety of alternative methods have been developed, such as the Delépine reactionDelepine reaction
The Delépine reaction is the organic synthesis of primary amines by reaction of a benzyl or alkyl halides with hexamethylenetetramine followed by acid hydrolysis of the quaternary ammonium salt...
, which uses hexamine
Hexamine
Hexamethylenetetramine is a heterocyclic organic compound with the formula 6N4. This white crystalline compound is highly soluble in water and polar organic solvents. It has a cage-like structure similar to adamantane. It is useful in the synthesis of other chemical compounds, e.g. plastics,...
. The Gabriel synthesis
Gabriel synthesis
The Gabriel synthesis is named for the German chemist Siegmund Gabriel. Traditionally, it is a chemical reaction that transforms primary alkyl halides into primary amines using potassium phthalimide....
, involving the use of an equivalent to NH2-, only applies to primary alkyl halides.