Amadori rearrangement
Encyclopedia
The amadori rearrangement is an organic reaction
describing the acid or base catalyzed
isomerization or rearrangement reaction
of the N-glycoside
of an aldose
or the glycosylamine
to the corresponding 1-amino-1-deoxy-ketose
. The reaction is important in carbohydrate chemistry
.
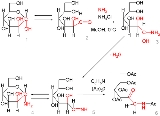
The reaction mechanism
is demonstrated starting from the reaction of D-mannose
in its closed (1) and open-form (2) with ammonia
the 1,1-amino-alcohol 3, which is unstable and loses water to the glycosylamine
(again the open imine
(5) and the closed form hemiaminal
(4)), which is the starting point for the actual Amadori rearrangement.
By treatment of the glycosylamine with pyridine
and acetic anhydride
, the imine group rearranges and the intermediate enol
, in turn, rearranges to the ketone
. In this particular reaction, all the alcohol
and amino groups are acylated
as well.
The reaction is associated with the Maillard reaction
in which the reagents are naturally occurring sugars and amino acid
s.
(AGE) as a result of glycation
.
The formation of an advanced glycation end-product involves the following steps:
The first two steps in this reaction are both reversible, but the last step is irreversible.
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
describing the acid or base catalyzed
Acid catalysis
In acid catalysis and base catalysis a chemical reaction is catalyzed by an acid or a base. The acid is often the proton and the base is often a hydroxyl ion. Typical reactions catalyzed by proton transfer are esterfications and aldol reactions. In these reactions the conjugate acid of the carbonyl...
isomerization or rearrangement reaction
Rearrangement reaction
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
of the N-glycoside
Glycoside
In chemistry, a glycoside is a molecule in which a sugar is bound to a non-carbohydrate moiety, usually a small organic molecule. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. These can be activated by enzyme...
of an aldose
Aldose
An aldose is a monosaccharide that contains only one aldehyde group per molecule. The chemical formula takes the form Cnn. The simplest possible aldose is the diose glycolaldehyde, which only contains two carbon atoms....
or the glycosylamine
Glycosylamine
Glycosylamine is a class of biochemical compounds consisting of an amine with a β-N-glycosidic bond to a carbohydrate, thus forming a cyclic hemiaminal ether bond .Examples include nucleosides such as adenosine....
to the corresponding 1-amino-1-deoxy-ketose
Ketose
A ketose is a sugar containing one ketone group per molecule.With 3 carbon atoms, dihydroxyacetone is the simplest of all ketoses and is the only one having no optical activity. Ketoses can isomerize into an aldose when the carbonyl group is located at the end of the molecule...
. The reaction is important in carbohydrate chemistry
Carbohydrate chemistry
Carbohydrate chemistry is a subdiscipline of chemistry primarily concerned with the synthesis, structure, and function of carbohydrate structures. Due to the general structure of carbohydrates, their synthesis is often preoccupied with the selective formation of glycosidic linkages and the...
.
The reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
is demonstrated starting from the reaction of D-mannose
Mannose
Mannose is a sugar monomer of the aldohexose series of carbohydrates. Mannose is a C-2 epimer of glucose. It is not part of human metabolism, but is a component of microbial cell walls, and is therefore a target of the immune system and also of antibiotics....
in its closed (1) and open-form (2) with ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
the 1,1-amino-alcohol 3, which is unstable and loses water to the glycosylamine
Glycosylamine
Glycosylamine is a class of biochemical compounds consisting of an amine with a β-N-glycosidic bond to a carbohydrate, thus forming a cyclic hemiaminal ether bond .Examples include nucleosides such as adenosine....
(again the open imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
(5) and the closed form hemiaminal
Hemiaminal
A hemiaminal is a functional group or type of chemical compound that has a hydroxyl group and an amine attached to the same carbon atom: -C-. R can be hydrogen or an alkyl group...
(4)), which is the starting point for the actual Amadori rearrangement.
By treatment of the glycosylamine with pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
and acetic anhydride
Acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula 2O. Commonly abbreviated Ac2O, it is the simplest isolatable acid anhydride and is a widely used reagent in organic synthesis...
, the imine group rearranges and the intermediate enol
Enol
Enols are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated anions of enols are called enolates...
, in turn, rearranges to the ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
. In this particular reaction, all the alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
and amino groups are acylated
Acylation
In chemistry, acylation is the process of adding an acyl group to a compound. The compound providing the acyl group is called the acylating agent....
as well.
The reaction is associated with the Maillard reaction
Maillard reaction
The Maillard reaction is a form of nonenzymatic browning similar to caramelization. It results from a chemical reaction between an amino acid and a reducing sugar, usually requiring heat....
in which the reagents are naturally occurring sugars and amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s.
Amadori product
An Amadori product is an intermediate in the production of an advanced glycation end-productAdvanced glycation endproduct
An advanced glycation end-product is the result of a chain of chemical reactions after an initial glycation reaction. The intermediate products are known, variously, as Amadori, Schiff base and Maillard products, named after the researchers who first described them. An advanced glycation...
(AGE) as a result of glycation
Glycation
Glycation is the result of the bonding of a protein or lipid molecule with a sugar molecule, such as fructose or glucose, without the controlling action of an enzyme. All blood sugars are reducing molecules. Glycation may occur either inside the body or outside the body...
.
The formation of an advanced glycation end-product involves the following steps:
- Formation of a Schiff baseSchiff baseA Schiff base, named after Hugo Schiff, is a compound with a functional group that contains a carbon-nitrogen double bond with the nitrogen atom connected to an aryl or alkyl group, not hydrogen....
: For example the aldehydeAldehydeAn aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
group of a glucoseGlucoseGlucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
molecule will combine with the amino group of a lysineLysineLysine is an α-amino acid with the chemical formula HO2CCH4NH2. It is an essential amino acid, which means that the human body cannot synthesize it. Its codons are AAA and AAG....
molecule (in a proteinProteinProteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
) to form an imineImineAn imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
or Schiff base, which is a double bondCovalent bondA covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
between the carbonCarbonCarbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
atomAtomThe atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
of the glucose and the nitrogenNitrogenNitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
atom of the lysine. - Formation of an Amadori product: The Amadori product is a re-arrangement from the Schiff base, wherein the hydrogenHydrogenHydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
atom from the hydroxylHydroxylA hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
group adjacent to the carbon-nitrogen double bond moves to bond to the nitrogen, leaving a ketoneKetoneIn organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
. - Formation of an advanced glycation end-product (AGE): The Amadori product is oxidized, most often by transition metalTransition metalThe term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
catalysisCatalysisCatalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
.
The first two steps in this reaction are both reversible, but the last step is irreversible.

