
Alveolar gas equation
Encyclopedia
The partial pressure
of oxygen (pO2) in the pulmonary alveoli is required to calculate both the alveolar-arterial gradient of oxygen and the amount of right-to-left cardiac shunt
, which are both clinically useful quantities. However it is not practical to take a sample of gas from the alveoli in order to directly measure the partial pressure of oxygen. The alveolar gas equation allows the calculation of the alveolar partial pressure of oxygen from data that is practically measurable. It was first characterized in 1946.
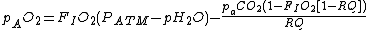
If FIO2 is small then

In which case the equation can be simplified to:
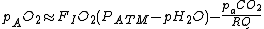
where:
Sample Values given for air at sea level at 37°C.
Partial pressure
In a mixture of ideal gases, each gas has a partial pressure which is the pressure which the gas would have if it alone occupied the volume. The total pressure of a gas mixture is the sum of the partial pressures of each individual gas in the mixture....
of oxygen (pO2) in the pulmonary alveoli is required to calculate both the alveolar-arterial gradient of oxygen and the amount of right-to-left cardiac shunt
Cardiac shunt
Cardiac shunts is when the blood flow follows a pattern in the heart that deviates from the normal circuit of the circulatory system. It may be described as right-left, left-to-right or bidirectional, or as systemic-to-pulmonary or pulmonary-to-systemic. The direction may be controlled by left...
, which are both clinically useful quantities. However it is not practical to take a sample of gas from the alveoli in order to directly measure the partial pressure of oxygen. The alveolar gas equation allows the calculation of the alveolar partial pressure of oxygen from data that is practically measurable. It was first characterized in 1946.
Assumptions
The equation relies on the following assumptions:- Inspired gas contains no carbon dioxide (CO2) or water
- Nitrogen (and any other gases except oxygen) in the inspired gas are in equilibrium with their dissolved states in the blood
- Inspired and alveolar gases obey the ideal gas lawIdeal gas lawThe ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. It was first stated by Émile Clapeyron in 1834 as a combination of Boyle's law and Charles's law...
- Carbon dioxide (CO2) in the alveolar gas is in equilibrium with the arterial blood i.e. that the alveolar and arterial partial pressures are equal
- The alveolar gas is saturated with water
Equation
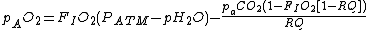
If FIO2 is small then

In which case the equation can be simplified to:
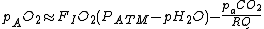
where:
| Quantity | Description | Sample value |
|---|---|---|
| pAO2 | The alveolar partial pressure of oxygen (pO2) | 107 mmHg (14.2 kPa) |
| FIO2 | The fraction of inspired gas that is oxygen (expressed as a decimal). | 0.21 |
| PATM | The prevailing atmospheric pressure | 760 mmHg (101 kPa) |
| pH2O | The saturated vapour pressure of water at body temperature and the prevailing atmospheric pressure | 47 mmHg (6.25 kPa) |
| paCO2 | The arterial partial pressure of carbon dioxide (pCO2) | 36 mmHg (4.79 kPa) |
| RQ (RER) | The respiratory quotient Respiratory quotient The respiratory quotient , is a unitless number used in calculations of basal metabolic rate when estimated from carbon dioxide production. Such measurements, like measurements of oxygen uptake, are forms of indirect calorimetry... (Respiratory Exchange Ratio) |
0.8 |
Sample Values given for air at sea level at 37°C.

