
Aluminium fluoride
Encyclopedia
Aluminium fluoride is an inorganic compound
used primarily in the production of aluminium
. This colourless solid can be prepared synthetically but also occurs in nature.
Alternatively, it is manufactured by thermal decomposition of ammonium hexafluoroaluminate. For small scale laboratory preparations, AlF3 can also be prepared by treating aluminium hydroxide
or aluminium metal with HF
.
Aluminium fluoride trihydrate is found in nature as the rare mineral rosenbergite.
adopts the rhenium trioxide
motif, featuring distorted AlF6 octahedra
. Each fluoride is connected to two Al centers. Because of it is a 3-dimensional polymer, AlF3 has a higher melting point
. In contrast, the other trihalides of aluminium, AlCl3
, AlBr3
, and AlI3
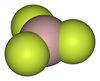
, which are either molecular or 1-dimensional polymers, have low melting points and evaporate readily to give dimers. In the gas phase, at ca. 1000 °C, aluminium fluoride exists as trigonal molecules of D3h symmetry
. The Al-F bond lengths of this gaseous molecule are 163 pm
.

lowers the melting point to below 1000 °C and increases the conductivity of the solution
. It is into this molten salt that aluminium oxide is dissolved and then electrolyzed to give bulk Al metal.
It is also used to inhibit fermentation
.
It is a sputtering target for preparation of low index films.
Inorganic compound
Inorganic compounds have traditionally been considered to be of inanimate, non-biological origin. In contrast, organic compounds have an explicit biological origin. However, over the past century, the classification of inorganic vs organic compounds has become less important to scientists,...
used primarily in the production of aluminium
Aluminium
Aluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances....
. This colourless solid can be prepared synthetically but also occurs in nature.
Production and occurrence
The majority of aluminium fluoride is mainly produced by treating alumina with hexafluorosilicic acid:- H2SiF6 + Al2O3 → 2 AlF3 + SiO2 + H2O
Alternatively, it is manufactured by thermal decomposition of ammonium hexafluoroaluminate. For small scale laboratory preparations, AlF3 can also be prepared by treating aluminium hydroxide
Aluminium hydroxide
Aluminium hydroxide, Al3, ATH, sometimes erroneously called Hydrate of alumina, is found in nature as the mineral gibbsite and its three, much more rare forms, polymorphs: bayerite, doyleite and nordstrandite. Closely related are aluminium oxide hydroxide, AlO, and aluminium oxide, Al2O3,...
or aluminium metal with HF
Hydrogen fluoride
Hydrogen fluoride is a chemical compound with the formula HF. This colorless gas is the principal industrial source of fluorine, often in the aqueous form as hydrofluoric acid, and thus is the precursor to many important compounds including pharmaceuticals and polymers . HF is widely used in the...
.
Aluminium fluoride trihydrate is found in nature as the rare mineral rosenbergite.
Structure
Its structureCrystal structure
In mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry...
adopts the rhenium trioxide
Rhenium trioxide
Rhenium trioxide is a red solid with a metallic lustre. It is the only stable trioxide of the Group 7 elements .-Structure:...
motif, featuring distorted AlF6 octahedra
Octahedron
In geometry, an octahedron is a polyhedron with eight faces. A regular octahedron is a Platonic solid composed of eight equilateral triangles, four of which meet at each vertex....
. Each fluoride is connected to two Al centers. Because of it is a 3-dimensional polymer, AlF3 has a higher melting point
Melting point
The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure...
. In contrast, the other trihalides of aluminium, AlCl3
Aluminium chloride
Aluminium chloride is the main compound of aluminium and chlorine. It is white, but samples are often contaminated with iron trichloride, giving it a yellow colour. The solid has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium metal, but large...
, AlBr3
Aluminium bromide
Aluminium bromide is any chemical compound with the empirical formula AlBrx. The species called "aluminium tribromide," is the most common aluminium bromide. The species aluminium monobromide forms from the reaction of HBr with Al metal at high temperature...
, and AlI3
Aluminium iodide
Aluminium iodide is any chemical compound containing only aluminium and iodine. Invariably, the name refers to a compound of the composition AlI3, formed by the reaction of aluminium and iodine or the action of HI on Al metal. The hexahydrate is obtained from a reaction between metallic aluminum...
, which are either molecular or 1-dimensional polymers, have low melting points and evaporate readily to give dimers. In the gas phase, at ca. 1000 °C, aluminium fluoride exists as trigonal molecules of D3h symmetry
Symmetry group
The symmetry group of an object is the group of all isometries under which it is invariant with composition as the operation...
. The Al-F bond lengths of this gaseous molecule are 163 pm
1 E-12 m
To help compare different orders of magnitude this page lists lengths between 10−12 m and 10−11 m .Distances shorter than 1 pm* 1 pm = 1 picometre = 1,000 femtometres* 1 pm = distance between atomic nuclei in a white dwarf...
.


Applications
Aluminium fluoride is an important additive for the production of aluminium by electrolysis. Together with cryoliteCryolite
Cryolite is an uncommon mineral identified with the once large deposit at Ivigtût on the west coast of Greenland, depleted by 1987....
lowers the melting point to below 1000 °C and increases the conductivity of the solution
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
. It is into this molten salt that aluminium oxide is dissolved and then electrolyzed to give bulk Al metal.
Niche uses
Together with zirconium fluoride, aluminium fluoride is an ingredient for the production of fluoroaluminate glasses.It is also used to inhibit fermentation
Fermentation (biochemistry)
Fermentation is the process of extracting energy from the oxidation of organic compounds, such as carbohydrates, using an endogenous electron acceptor, which is usually an organic compound. In contrast, respiration is where electrons are donated to an exogenous electron acceptor, such as oxygen,...
.
It is a sputtering target for preparation of low index films.

