
Alliinase
Encyclopedia
In enzymology, an alliin lyase is an enzyme
that catalyzes
the chemical reaction
Hence, this enzyme has one substrate
, S-alkyl-L-cysteine
S-oxide
, and two products
, alkyl sulfenate
and 2-aminoacrylate
.
This enzyme belongs to the family of lyase
s, specifically the class of carbon-sulfur lyases. The systematic name of this enzyme class is S-alkyl-L-cysteine S-oxide alkyl-sulfenate-lyase (2-aminoacrylate-forming). Other names in common use include alliinase, cysteine sulfoxide lyase, alkylcysteine sulfoxide lyase, S-alkylcysteine sulfoxide lyase, L-cysteine sulfoxide lyase, S-alkyl-L-cysteine sulfoxide lyase, and alliin alkyl-sulfenate-lyase. It employs one cofactor
, pyridoxal phosphate.
Many alliinases contain a novel N-terminal epidermal growth factor-like domain (EGF-like domain
).
, such as garlic and onions. Alliinase is responsible for catalyzing
chemical reactions that produce the volatile chemicals that give these foods their flavors, odors, and tear-inducing properties. Alliinases are part of the plant's defense against herbivores. Alliinase is normally sequestered within a plant cell, but, when the plant is damaged by a feeding animal, the alliinase is released to catalyze the production of the pungent chemicals. This tends to have a deterrent effect on the animal. The same reaction occurs when onion or garlic is cut with a knife in the kitchen.
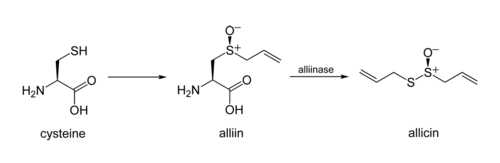
converting it into allicin. The process involves two stages: elimination of allyl sulfenic acid from the amino-acid unit (with α-aminoacrylic acid as a byproduct), and then condensation of two of the sulfinic-acid molecules.
 Alliin and related substrates found in nature are chiral
Alliin and related substrates found in nature are chiral
at the sulfoxide
position (usually having the S absolute configuration, and alliin itself was the first natural product found to have both carbon- and sulfur-centered stereochemistry. However, the sulfenic acid intermediate is not chiral, and the final product's stereochemistry is not controlled.
There are a range of similar enzymes that can react with the cysteine-derived sulfoxides present in different species. In onions, an isomer of alliin is converted to1-propenesulfenic acid. A separate enzyme then converts this chemical to syn-propanethial-S-oxide
, a potent lachrymator. The analogous butyl
compound has been found in another Allium species.
have been solved for this class of enzymes, using X-ray crystallography
. The PDB
accession codes are , , and .
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that catalyzes
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
- an S-alkyl-L-cysteine S-oxide
 an alkyl sulfenate + 2-aminoacrylate
an alkyl sulfenate + 2-aminoacrylate
Hence, this enzyme has one substrate
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
, S-alkyl-L-cysteine
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
S-oxide
Sulfoxide
A sulfoxide is a chemical compound containing a sulfinyl functional group attached to two carbon atoms. Sulfoxides can be considered as oxidized sulfides...
, and two products
Product (chemistry)
Product are formed during chemical reactions as reagents are consumed. Products have lower energy than the reagents and are produced during the reaction according to the second law of thermodynamics. The released energy comes from changes in chemical bonds between atoms in reagent molecules and...
, alkyl sulfenate
Sulfenic acid
A sulfenic acid is an organosulfur compound and oxoacid with the general formula RSOH, where R ≠ H. Simple sulfenic acids, such as methanesulfenic acid, CH3SOH, are highly reactive and cannot be isolated in solution. In the gas phase the lifetime of methanesulfenic acid is about one minute...
and 2-aminoacrylate
Acrylic acid
Acrylic acid is an organic compound with the formula CH2=CHCO2H. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a characteristic acrid or tart smell. It is miscible with water, alcohols,...
.
This enzyme belongs to the family of lyase
Lyase
In biochemistry, a lyase is an enzyme that catalyzes the breaking of various chemical bonds by means other than hydrolysis and oxidation, often forming a new double bond or a new ring structure...
s, specifically the class of carbon-sulfur lyases. The systematic name of this enzyme class is S-alkyl-L-cysteine S-oxide alkyl-sulfenate-lyase (2-aminoacrylate-forming). Other names in common use include alliinase, cysteine sulfoxide lyase, alkylcysteine sulfoxide lyase, S-alkylcysteine sulfoxide lyase, L-cysteine sulfoxide lyase, S-alkyl-L-cysteine sulfoxide lyase, and alliin alkyl-sulfenate-lyase. It employs one cofactor
Cofactor (biochemistry)
A cofactor is a non-protein chemical compound that is bound to a protein and is required for the protein's biological activity. These proteins are commonly enzymes, and cofactors can be considered "helper molecules" that assist in biochemical transformations....
, pyridoxal phosphate.
Many alliinases contain a novel N-terminal epidermal growth factor-like domain (EGF-like domain
EGF-like domain
EGF-like domain is an evolutionary conserved protein domain, of about thirty to forty amino-acid residues long, which was found in a large number of mostly animal proteins. All these repeats are found in the extracellular domain of membrane-bound proteins or in proteins known to be secreted...
).
Occurrence
These enzymes are found in plants of the genus AlliumAllium
Allium is a monocot genus of flowering plants, informally referred to as the onion genus. The generic name Allium is the Latin word for garlic....
, such as garlic and onions. Alliinase is responsible for catalyzing
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
chemical reactions that produce the volatile chemicals that give these foods their flavors, odors, and tear-inducing properties. Alliinases are part of the plant's defense against herbivores. Alliinase is normally sequestered within a plant cell, but, when the plant is damaged by a feeding animal, the alliinase is released to catalyze the production of the pungent chemicals. This tends to have a deterrent effect on the animal. The same reaction occurs when onion or garlic is cut with a knife in the kitchen.
Chemistry
In garlic, an alliinase enzyme acts on the chemical alliinAlliin
Alliin is a sulfoxide that is a natural constituent of fresh garlic. It is a derivative of the amino acid cysteine. When fresh garlic is chopped or crushed, the enzyme alliinase converts alliin into allicin, which is responsible for the aroma of fresh garlic...
converting it into allicin. The process involves two stages: elimination of allyl sulfenic acid from the amino-acid unit (with α-aminoacrylic acid as a byproduct), and then condensation of two of the sulfinic-acid molecules.

Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
at the sulfoxide
Sulfoxide
A sulfoxide is a chemical compound containing a sulfinyl functional group attached to two carbon atoms. Sulfoxides can be considered as oxidized sulfides...
position (usually having the S absolute configuration, and alliin itself was the first natural product found to have both carbon- and sulfur-centered stereochemistry. However, the sulfenic acid intermediate is not chiral, and the final product's stereochemistry is not controlled.
There are a range of similar enzymes that can react with the cysteine-derived sulfoxides present in different species. In onions, an isomer of alliin is converted to1-propenesulfenic acid. A separate enzyme then converts this chemical to syn-propanethial-S-oxide
Syn-propanethial-S-oxide
syn-Propanethial S-oxide is a gas that acts as a lachrymatory agent . The chemical is released from onions, Allium cepa, as they are sliced...
, a potent lachrymator. The analogous butyl
Butyl
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula -C4H9, derived from either of the two isomers of butane....
compound has been found in another Allium species.
Structural studies
As of late 2007, 3 structuresTertiary structure
In biochemistry and molecular biology, the tertiary structure of a protein or any other macromolecule is its three-dimensional structure, as defined by the atomic coordinates.-Relationship to primary structure:...
have been solved for this class of enzymes, using X-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
. The PDB
Protein Data Bank
The Protein Data Bank is a repository for the 3-D structural data of large biological molecules, such as proteins and nucleic acids....
accession codes are , , and .

