
Aldol-Tishchenko reaction
Encyclopedia
The Aldol-Tishchenko reaction is a tandem reaction involving an Aldol reaction
and a Tishchenko reaction
. In organic synthesis
it is a method to convert aldehyde
s and ketone
s into 1,3-hydroxyl
compounds. The reaction sequence in many examples starts from conversion of a ketone into an enolate by action of Lithium diisopropylamide
(LDA). The mono-ester diol is then converted into the diol by a hydrolysis
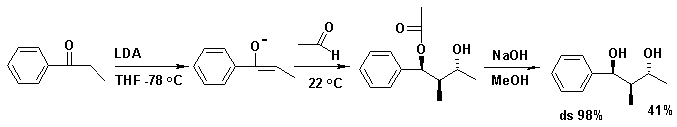
step. With both the acetyl trimethylsilane [1] and propiophenone [2] as reactants, the diol is obtained as a pure diastereoisomer.

Aldol reaction
The aldol reaction is a powerful means of forming carbon–carbon bonds in organic chemistry.Discovered independently by Charles-Adolphe Wurtz and Alexander Porfyrevich Borodin in 1872, the reaction combines two carbonyl compounds to form a new β-hydroxy carbonyl compound...
and a Tishchenko reaction
Tishchenko reaction
The Tishchenko reaction is a chemical reaction that involves disproportionation of an aldehyde lacking a hydrogen atom in the alpha position in the presence of an alkoxide. The reaction product is an ester. Catalysts are aluminium alkoxides or sodium alkoxides...
. In organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
it is a method to convert aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s and ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s into 1,3-hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
compounds. The reaction sequence in many examples starts from conversion of a ketone into an enolate by action of Lithium diisopropylamide
Lithium diisopropylamide
Lithium diisopropylamide is the chemical compound with the formula [2CH]2NLi. Generally abbreviated LDA, it is a strong base used in organic chemistry for the deprotonation of weakly acidic compounds. The reagent has been widely accepted because it is soluble in non-polar organic solvents and it...
(LDA). The mono-ester diol is then converted into the diol by a hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
step. With both the acetyl trimethylsilane [1] and propiophenone [2] as reactants, the diol is obtained as a pure diastereoisomer.



