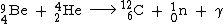Actinium
Encyclopedia
Actinium is a radioactive chemical element
with the symbol Ac and atomic number
89, which was discovered in 1899. It was the first non-primordial radioactive element to be isolated. Polonium
, radium
and radon
were observed before actinium, but they were not isolated until 1902. Actinium gave the name to the actinide
series, a group of 15 similar elements between actinium and lawrencium
in the periodic table
.
A soft, silvery-white radioactive metal, actinium reacts rapidly with oxygen and moisture in air forming a white coating of actinium oxide that prevents further oxidation. As most lanthanide
s and actinides, actinium assumes oxidation state
+3 in nearly all its chemical compounds. Actinium is found only in traces in uranium
ores as 227Ac isotope
, which decays with a half-life
of 21.773 years, predominantly emitting beta particle
s. One tonne
of uranium
ore contains about 0.2 milligrams of actinium. The close similarity of physical and chemical properties of actinium and lanthanum
makes separation of actinium from the ore impractical. Instead, the element is prepared, in milligram amounts, by the neutron irradiation of 226 in a nuclear reactor
. Owing to its scarcity, high price and radioactivity, actinium has no significant industrial use. Its current applications include a neutron source and an agent for radiation therapy
targeting cancer cells in the body.
, a French chemist, announced the discovery of a new element in 1899. He separated it from pitchblende
residues left by Marie
and Pierre Curie
after they had extracted radium
. In 1899, Debierne described the substance as similar to titanium
and (in 1900) as similar to thorium
. Friedrich Oskar Giesel
independently discovered actinium in 1902 as a substance being similar to lanthanum
and called it "emanium" in 1904. After a comparison of substances in 1904, Debierne's name was retained because it had seniority.
The stated history of the discovery of actinium remained uncertain for decades. Articles published in the 1970s and later suggest that Debierne's results published in 1904 conflict with those reported in 1899 and 1900. Whether Debierne and Giesel should share the merit of discovery or if Giesel alone should be credited is still under debate.
The name actinium originates from the Ancient Greek
aktis, aktinos (ακτίς, ακτίνος), meaning beam or ray. Its symbol Ac is also used in abbreviations of other compounds that have nothing to do with actinium, such as acetyl
, acetate
and sometimes acetaldehyde
.
. Owing to its strong radioactivity, actinium glows in the dark with a pale blue light, which originates from the surrounding air ionized by the energetic particles emitted from actinium. Actinium has similar chemical properties as lanthanum
and other lanthanides, and therefore these elements are difficult to separate when extracting from uranium ores. Solvent extraction and ion chromatography are commonly used for the separation.
The first element of the actinide
s, actinium gave the group its name, much as lanthanum
has done for the lanthanide
s. The group of elements is more diverse than the lanthanides and therefore it was not until 1945 that Glenn T. Seaborg
proposed the most significant change to Mendeleev's periodic table
, by introducing the actinides.
Actinium reacts rapidly with oxygen and moisture in air forming a white coating of actinium oxide that prevents further oxidation. As with most lanthanides and actinides, actinium exists in the oxidation state
+3, and the Ac3+ ions are colorless in solutions. The oxidation state +3 originates from the 6d17s2 electronic configuration of actinium, that is it easily donates 3 electrons assuming a stable closed-shell structure of the noble gas
radon
. The oxidation state +2 is only known for actinium dihydride.
Here a, b and c are lattice constants, No is space group number and Z is the number of formula unit
s per unit cell. Density was not measured directly but calculated from the lattice parameters.
at 1100 °C, in vacuum. It crystal lattice is isotypic with the oxides of most trivalent rare-earth metals.
to a solution containing actinium ions. In the latter method, actinium metal is treated with hydrogen fluoride vapors at 700 °C in an all-platinum setup. Treating actinium trifluoride with ammonium hydroxide
at 900–1000 °C yields oxyfluoride AcOF. Whereas lanthanum oxyfluoride can be easily obtained by burning lanthanum trifluoride in air at 800 °C for an hour, similar treatment of actinium trifluoride yields no AcOF and only results in melting of the initial product.
Actinium trichloride is obtained by reacting actinium hydroxide or oxalate
with carbon tetrachloride
vapors at temperatures above 960 °C. Similar to oxyfluoride, actinium oxychloride can be prepared by hydrolyzing actinium trichloride with ammonium hydroxide
at 1000 °C. However, in contrast to the oxyfluoride, the oxychloride could well be synthesized by igniting a solution of actinium trichloride in hydrochloric acid
with ammonia
.
Reaction of aluminium bromide
and actinium oxide yields actinium tribromide:
and treating it with ammonium hydroxide at 500 °C results in the oxybromide AcOBr.
Mixing monosodium phosphate
(NaH2PO4) with a solution of actinium in hydrochloric acid yields white-colored actinium phosphate hemihydrate (AcPO4·0.5H2O), and heating actinium oxalate with hydrogen sulfide
vapors at 1400 °C for a few minutes results in a black actinium sulfide Ac2S3. It may possibly be produced by acting with a mixture of hydrogen sulfide and carbon disulfide on actinium oxide at 1000 °C.
; . Thirty-six radioisotopes have been identified, the most stable being with a half-life
of 21.772 years, with a half-life of 10.0 days and with a half-life of 29.37 hours. All remaining radioactive
isotopes have half-lives that are less than 10 hours and the majority of them have half-lives shorter than 1 minute. The shortest-lived known isotope of actinium is (half-life of 69 nanoseconds) which decays through alpha decay
and electron capture
. Actinium also has two meta states.
Purified comes into equilibrium with its decay products at the end of 185 days. It decays according to its 21.773-year half-life emitting mostly beta (98.8%) and some alpha particles (1.2%); the successive decay products are part of the actinium series. Owing to the low available amounts, low energy of its beta particles (46 keV) and low intensity of alpha radiation, is difficult to detect directly by its emission and it is therefore traced via its decay products. The isotopes of actinium range in atomic weight
from 206 u
to 236 u .
!Isotope
!Production
!Decay
!Half-life
|-
|221Ac
|232Th(d,9n)225Pa(α)→221Ac
|α
|52 ms
|-
|222Ac
|232Th(d,8n)226Pa(α)→222Ac
|α
|5.0 s
|-
|223Ac
|232Th(d,7n)227Pa(α)→223Ac
|α
|2.1 min
|-
|224Ac
|232Th(d,6n)228Pa(α)→224Ac
|α
|2.78 hours
|-
|225Ac
|232Th(n,γ)233Th(β−)→233Pa(β−)→233U(α)→229Th(α)→225Ra(β−)225Ac
|α
|10 days
|-
|226Ac
|226Ra(d,2n)226Ac
|α, β−
electron capture
|29.37 hours
|-
|227Ac
|235U(α)→231Th(β−)→231Pa(α)→227Ac
|α, β−
|21.77 years
|-
|228Ac
|232Th(α)→228Ra(β−)→228Ac
|β−
|6.15 hours
|-
|229Ac
|228Ra(n,γ)229Ra(β−)→229Ac
|β−
|62.7 min
|-
|230Ac
|232Th(d,α)230Ac
|β−
|122 s
|-
|231Ac
|232Th(γ,p)231Ac
|β−
|7.5 min
|-
|232Ac
|232Th(n,p)232Ac
|β−
|119 s
|}>
ores as 227Ac – one tonne of ore contains about 0.2 milligrams of actinium. These uranium ores sometimes contain lanthanum
and other lanthanide
s. The actinium isotope
227Ac is a transient member of the actinium series decay chain
, which begins with the parent isotope 235U
(or 239Pu
) and ends with the stable lead isotope 207Pb
. Another actinium isotope (225Ac) is transiently present in the neptunium series decay chain
, beginning with 237Np
(or 233U)
and ending with near-stable bismuth
(209Bi).
The low natural concentration, and the close similarity of physical and chemical properties to those of lanthanum and other lanthanides, which are always abundant in actinium-bearing ores, render separation of actinium from the ore impractical, and complete separation was never achieved. Instead, actinium is prepared, in milligram amounts, by the neutron irradiation of 226 in a nuclear reactor
.

The reaction yield is about 2% of the radium weight. 227Ac can further capture neutrons resulting in small amounts of 228Ac. After the synthesis, actinium is separated from radium and from the products of decay and nuclear fusion, such as thorium, polonium, lead and bismuth. The extraction can be performed with thenoyltrifluoroacetone-benzene
solution from an aqueous solution of the radiation products, and the selectivity to a certain element is achieved by adjusting the pH
(to about 6.0 for actinium). An alternative procedure is anion exchange with an appropriate resin
in nitric acid
, which can result in a separation factor of 1,000,000 for radium and actinium vs. thorium in a two-stage process. Actinium can then be separated from radium, with a ratio of about 100, using a low cross-linking cation exchange resin and nitric acid as eluant.
225Ac was first produced artificially at the Institute for Transuranium Elements
(ITU) in Germany using a cyclotron
and at St George Hospital
in Sydney using a linac
in 2000. This rare isotope has potential applications in radiation therapy and is most efficiently produced by bombarding a radium-226 target with 20–30 MeV deuterium
ions. This reaction also yields 226Ac which however decays with a half-life of 29 hours and thus does not contaminate 225Ac.
Actinium metal has been prepared by the reduction of actinium fluoride with lithium
vapor in vacuum at a temperature between 1100 and 1300 °C. Higher temperatures resulted in evaporation of the product and lower ones lead to an incomplete transformation. Lithium was chosen among other alkali metals because its fluoride is most volatile.
227Ac is highly radioactive and was therefore studied for use as an active element of radioisotope thermoelectric generator
s, for example in spacecraft. The oxide of 227Ac pressed with beryllium
is also an efficient neutron source
with the activity exceeding that of the standard americium-beryllium and radium-beryllium pairs. In all those applications, 227Ac (a beta source) is merely a progenitor which generates alpha-emitting isotopes upon its decay. Beryllium captures alpha particles and emits neutrons owing to its large cross-section for the (α,n) nuclear reaction:
The 227AcBe neutron sources can be applied in a neutron probe
– a standard device for measuring the quantity of water present in soil, as well as moisture/density for quality control in highway construction. Such probes are also used in well logging applications, in neutron radiography
, tomography and other radiochemical investigations.
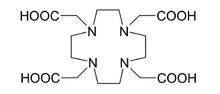 225Ac is applied in medicine to produce 213 in a reusable generator or can be used alone as an agent for radiation therapy
225Ac is applied in medicine to produce 213 in a reusable generator or can be used alone as an agent for radiation therapy
, in particular targeted alpha therapy (TAT). This isotope has a half-life of 10 days that makes it much more suitable for radiation therapy than 213Bi (half-life 46 minutes). Not only 225Ac itself, but also its decay products emit alpha particles which kill cancer cells in the body. The major difficulty with application of 225Ac was that intravenous injection of simple actinium complexes resulted in their accumulation in the bones and liver for a period of tens of years. As a result, after the cancer cells were quickly killed by alpha particles from 225Ac, the radiation from the actinium and its decay products might induce new mutations. To solve this problem, 225Ac was bound to a chelating
agent, such as citrate
, ethylenediaminetetraacetic acid (EDTA) or diethylene triamine pentaacetic acid (DTPA). This reduced actinium accumulation in the bones, but the excretion from the body remained slow. Much better results were obtained with such chelating agents as HEHA or DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) coupled to trastuzumab
, a monoclonal antibody that interferes with the HER2/neu
receptor
. The latter delivery combination was tested on mice and proved to be effective against leukemia
, lymphoma
, breast
, ovarian
, neuroblastoma
and prostate cancer
s.
The medium half-life of 227Ac (21.77 years) makes it very convenient radioactive isotope in modeling the slow vertical mixing of oceanic waters. The associated processes cannot be studied with the required accuracy by direct measurements of current velocities (of the order 50 meters per year). However, evaluation of the concentration depth-profiles for different isotopes allows estimating the mixing rates. The physics behind this method is as follows: oceanic waters contain homogeneously dispersed 235U. Its decay product, 231Pa, gradually precipitates to the bottom, so that its concentration first increases with depth and then stays nearly constant. 231Pa decays to 227Ac; however, the concentration of the latter isotope does not follow the 231Pa depth profile, but instead increases toward the sea bottom. This occurs because of the mixing processes which raise some additional 227Ac from the sea bottom. Thus analysis of both 231Pa and 227Ac depth profiles allows to model the mixing behavior.
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
with the symbol Ac and atomic number
Atomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
89, which was discovered in 1899. It was the first non-primordial radioactive element to be isolated. Polonium
Polonium
Polonium is a chemical element with the symbol Po and atomic number 84, discovered in 1898 by Marie Skłodowska-Curie and Pierre Curie. A rare and highly radioactive element, polonium is chemically similar to bismuth and tellurium, and it occurs in uranium ores. Polonium has been studied for...
, radium
Radium
Radium is a chemical element with atomic number 88, represented by the symbol Ra. Radium is an almost pure-white alkaline earth metal, but it readily oxidizes on exposure to air, becoming black in color. All isotopes of radium are highly radioactive, with the most stable isotope being radium-226,...
and radon
Radon
Radon is a chemical element with symbol Rn and atomic number 86. It is a radioactive, colorless, odorless, tasteless noble gas, occurring naturally as the decay product of uranium or thorium. Its most stable isotope, 222Rn, has a half-life of 3.8 days...
were observed before actinium, but they were not isolated until 1902. Actinium gave the name to the actinide
Actinide
The actinide or actinoid series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium.The actinide series derives its name from the group 3 element actinium...
series, a group of 15 similar elements between actinium and lawrencium
Lawrencium
Lawrencium is a radioactive synthetic chemical element with the symbol Lr and atomic number 103. In the periodic table of the elements, it is a period 7 d-block element and the last element of actinide series...
in the periodic table
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
.
A soft, silvery-white radioactive metal, actinium reacts rapidly with oxygen and moisture in air forming a white coating of actinium oxide that prevents further oxidation. As most lanthanide
Lanthanide
The lanthanide or lanthanoid series comprises the fifteen metallic chemical elements with atomic numbers 57 through 71, from lanthanum through lutetium...
s and actinides, actinium assumes oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
+3 in nearly all its chemical compounds. Actinium is found only in traces in uranium
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
ores as 227Ac isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
, which decays with a half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of 21.773 years, predominantly emitting beta particle
Beta particle
Beta particles are high-energy, high-speed electrons or positrons emitted by certain types of radioactive nuclei such as potassium-40. The beta particles emitted are a form of ionizing radiation also known as beta rays. The production of beta particles is termed beta decay...
s. One tonne
Tonne
The tonne, known as the metric ton in the US , often put pleonastically as "metric tonne" to avoid confusion with ton, is a metric system unit of mass equal to 1000 kilograms. The tonne is not an International System of Units unit, but is accepted for use with the SI...
of uranium
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
ore contains about 0.2 milligrams of actinium. The close similarity of physical and chemical properties of actinium and lanthanum
Lanthanum
Lanthanum is a chemical element with the symbol La and atomic number 57.Lanthanum is a silvery white metallic element that belongs to group 3 of the periodic table and is the first element of the lanthanide series. It is found in some rare-earth minerals, usually in combination with cerium and...
makes separation of actinium from the ore impractical. Instead, the element is prepared, in milligram amounts, by the neutron irradiation of 226 in a nuclear reactor
Nuclear reactor
A nuclear reactor is a device to initiate and control a sustained nuclear chain reaction. Most commonly they are used for generating electricity and for the propulsion of ships. Usually heat from nuclear fission is passed to a working fluid , which runs through turbines that power either ship's...
. Owing to its scarcity, high price and radioactivity, actinium has no significant industrial use. Its current applications include a neutron source and an agent for radiation therapy
Radiation therapy
Radiation therapy , radiation oncology, or radiotherapy , sometimes abbreviated to XRT or DXT, is the medical use of ionizing radiation, generally as part of cancer treatment to control malignant cells.Radiation therapy is commonly applied to the cancerous tumor because of its ability to control...
targeting cancer cells in the body.
History
André-Louis DebierneAndré-Louis Debierne
André-Louis Debierne was a French chemist and is considered the discoverer of the element actinium....
, a French chemist, announced the discovery of a new element in 1899. He separated it from pitchblende
Uraninite
Uraninite is a radioactive, uranium-rich mineral and ore with a chemical composition that is largely UO2, but also contains UO3 and oxides of lead, thorium, and rare earth elements...
residues left by Marie
Marie Curie
Marie Skłodowska-Curie was a physicist and chemist famous for her pioneering research on radioactivity. She was the first person honored with two Nobel Prizes—in physics and chemistry...
and Pierre Curie
Pierre Curie
Pierre Curie was a French physicist, a pioneer in crystallography, magnetism, piezoelectricity and radioactivity, and Nobel laureate. He was the son of Dr. Eugène Curie and Sophie-Claire Depouilly Curie ...
after they had extracted radium
Radium
Radium is a chemical element with atomic number 88, represented by the symbol Ra. Radium is an almost pure-white alkaline earth metal, but it readily oxidizes on exposure to air, becoming black in color. All isotopes of radium are highly radioactive, with the most stable isotope being radium-226,...
. In 1899, Debierne described the substance as similar to titanium
Titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. It has a low density and is a strong, lustrous, corrosion-resistant transition metal with a silver color....
and (in 1900) as similar to thorium
Thorium
Thorium is a natural radioactive chemical element with the symbol Th and atomic number 90. It was discovered in 1828 and named after Thor, the Norse god of thunder....
. Friedrich Oskar Giesel
Friedrich Oskar Giesel
Friedrich Oskar Giesel was a German organic chemist. During his work in a quinine factory in the late 1890s he started to work on the at-that-time-new field of radiochemistry and started the production of radium. In the period between 1902 and 1904 he was able to isolate a new element emanium...
independently discovered actinium in 1902 as a substance being similar to lanthanum
Lanthanum
Lanthanum is a chemical element with the symbol La and atomic number 57.Lanthanum is a silvery white metallic element that belongs to group 3 of the periodic table and is the first element of the lanthanide series. It is found in some rare-earth minerals, usually in combination with cerium and...
and called it "emanium" in 1904. After a comparison of substances in 1904, Debierne's name was retained because it had seniority.
The stated history of the discovery of actinium remained uncertain for decades. Articles published in the 1970s and later suggest that Debierne's results published in 1904 conflict with those reported in 1899 and 1900. Whether Debierne and Giesel should share the merit of discovery or if Giesel alone should be credited is still under debate.
The name actinium originates from the Ancient Greek
Ancient Greek
Ancient Greek is the stage of the Greek language in the periods spanning the times c. 9th–6th centuries BC, , c. 5th–4th centuries BC , and the c. 3rd century BC – 6th century AD of ancient Greece and the ancient world; being predated in the 2nd millennium BC by Mycenaean Greek...
aktis, aktinos (ακτίς, ακτίνος), meaning beam or ray. Its symbol Ac is also used in abbreviations of other compounds that have nothing to do with actinium, such as acetyl
Acetyl
In organic chemistry, acetyl is a functional group, the acyl with chemical formula COCH3. It is sometimes represented by the symbol Ac . The acetyl group contains a methyl group single-bonded to a carbonyl...
, acetate
Acetate
An acetate is a derivative of acetic acid. This term includes salts and esters, as well as the anion found in solution. Most of the approximately 5 billion kilograms of acetic acid produced annually in industry are used in the production of acetates, which usually take the form of polymers. In...
and sometimes acetaldehyde
Acetaldehyde
Acetaldehyde is an organic chemical compound with the formula CH3CHO or MeCHO. It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale industrially. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants as part...
.
Properties
Actinium is a soft, silvery-white, radioactive, metallic element. Its estimated shear modulus is similar to that of leadLead
Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
. Owing to its strong radioactivity, actinium glows in the dark with a pale blue light, which originates from the surrounding air ionized by the energetic particles emitted from actinium. Actinium has similar chemical properties as lanthanum
Lanthanum
Lanthanum is a chemical element with the symbol La and atomic number 57.Lanthanum is a silvery white metallic element that belongs to group 3 of the periodic table and is the first element of the lanthanide series. It is found in some rare-earth minerals, usually in combination with cerium and...
and other lanthanides, and therefore these elements are difficult to separate when extracting from uranium ores. Solvent extraction and ion chromatography are commonly used for the separation.
The first element of the actinide
Actinide
The actinide or actinoid series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium.The actinide series derives its name from the group 3 element actinium...
s, actinium gave the group its name, much as lanthanum
Lanthanum
Lanthanum is a chemical element with the symbol La and atomic number 57.Lanthanum is a silvery white metallic element that belongs to group 3 of the periodic table and is the first element of the lanthanide series. It is found in some rare-earth minerals, usually in combination with cerium and...
has done for the lanthanide
Lanthanide
The lanthanide or lanthanoid series comprises the fifteen metallic chemical elements with atomic numbers 57 through 71, from lanthanum through lutetium...
s. The group of elements is more diverse than the lanthanides and therefore it was not until 1945 that Glenn T. Seaborg
Glenn T. Seaborg
Glenn Theodore Seaborg was an American scientist who won the 1951 Nobel Prize in Chemistry for "discoveries in the chemistry of the transuranium elements", contributed to the discovery and isolation of ten elements, and developed the actinide concept, which led to the current arrangement of the...
proposed the most significant change to Mendeleev's periodic table
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
, by introducing the actinides.
Actinium reacts rapidly with oxygen and moisture in air forming a white coating of actinium oxide that prevents further oxidation. As with most lanthanides and actinides, actinium exists in the oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
+3, and the Ac3+ ions are colorless in solutions. The oxidation state +3 originates from the 6d17s2 electronic configuration of actinium, that is it easily donates 3 electrons assuming a stable closed-shell structure of the noble gas
Noble gas
The noble gases are a group of chemical elements with very similar properties: under standard conditions, they are all odorless, colorless, monatomic gases, with very low chemical reactivity...
radon
Radon
Radon is a chemical element with symbol Rn and atomic number 86. It is a radioactive, colorless, odorless, tasteless noble gas, occurring naturally as the decay product of uranium or thorium. Its most stable isotope, 222Rn, has a half-life of 3.8 days...
. The oxidation state +2 is only known for actinium dihydride.
Chemical compounds
Only a limited number of actinium compounds are known including AcF3, AcCl3, AcBr3, AcOF, AcOCl, AcOBr, Ac2S3, Ac2O3 and AcPO4. Except for AcPO4, they are all similar to the corresponding lanthanum compounds and contain actinium in the oxidation state +3. In particular, the lattice constants of the analogous lanthanum and actinium compounds differ by only a few percent.| Formula | color | symmetry | space group Space group In mathematics and geometry, a space group is a symmetry group, usually for three dimensions, that divides space into discrete repeatable domains.In three dimensions, there are 219 unique types, or counted as 230 if chiral copies are considered distinct... |
No | Pearson symbol Pearson symbol The Pearson symbol, or Pearson notation, is used in crystallography as a means of describing a crystal structure, and was originated by W.B. Pearson. The symbol is made up of two letters followed by a number. For example:* Diamond structure, cF8... |
a (pm) | b (pm) | c (pm) | Z | density, g/cm3 |
|---|---|---|---|---|---|---|---|---|---|---|
| Ac | silvery | fcc Cubic crystal system In crystallography, the cubic crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.... |
Fmm | 225 | cF4 | 531.1 | 531.1 | 531.1 | 4 | 10.07 |
| AcH2 | cubic | Fmm | 225 | cF12 | 567 | 567 | 567 | 4 | 8.35 | |
| Ac2O3 | white | trigonal | Pm1 | 164 | hP5 | 408 | 408 | 630 | 1 | 9.18 |
| Ac2S3 | cubic | I3d | 220 | cI28 | 778.56 | 778.56 | 778.56 | 4 | 6.71 | |
| AcF3 | white | hexagonal Hexagonal crystal system In crystallography, the hexagonal crystal system is one of the 7 crystal systems, the hexagonal lattice system is one of the 7 lattice systems, and the hexagonal crystal family is one of the 6 crystal families... |
Pc1 | 165 | hP24 | 741 | 741 | 755 | 6 | 7.88 |
| AcCl3 | hexagonal | P63/m | 165 | hP8 | 764 | 764 | 456 | 2 | 4.8 | |
| AcBr3 | white | hexagonal | P63/m | 165 | hP8 | 764 | 764 | 456 | 2 | 5.85 |
| AcOF | white | cubic | Fmm | 593.1 | 8.28 | |||||
| AcOCl | tetragonal Tetragonal crystal system In crystallography, the tetragonal crystal system is one of the 7 lattice point groups. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the cube becomes a rectangular prism with a square base and height .There are two tetragonal Bravais... |
424 | 424 | 707 | 7.23 | |||||
| AcOBr | tetragonal | 427 | 427 | 740 | 7.89 | |||||
| AcPO4·0.5H2O | hexagonal | 721 | 721 | 664 | 5.48 |
Here a, b and c are lattice constants, No is space group number and Z is the number of formula unit
Formula unit
A formula unit in chemistry is the empirical formula of an ionic or covalent network solid compound used as an independent entity for stoichiometric calculations. It is the lowest whole number ratio of ions represented in an ionic compound...
s per unit cell. Density was not measured directly but calculated from the lattice parameters.
Oxides
Actinium oxide (Ac2O3) can be obtained by heating the hydroxide at 500 °C or the oxalateOxalate
Oxalate , is the dianion with formula C2O42− also written 22−. Either name is often used for derivatives, such as disodium oxalate, 2C2O42−, or an ester of oxalic acid Oxalate (IUPAC: ethanedioate), is the dianion with formula C2O42− also written (COO)22−. Either...
at 1100 °C, in vacuum. It crystal lattice is isotypic with the oxides of most trivalent rare-earth metals.
Halides
Actinium trifluoride can be produced either in solution or in solid reaction. The former reaction is carried out at room temperature, by adding hydrofluoric acidHydrofluoric acid
Hydrofluoric acid is a solution of hydrogen fluoride in water. It is a valued source of fluorine and is the precursor to numerous pharmaceuticals such as fluoxetine and diverse materials such as PTFE ....
to a solution containing actinium ions. In the latter method, actinium metal is treated with hydrogen fluoride vapors at 700 °C in an all-platinum setup. Treating actinium trifluoride with ammonium hydroxide
Ammonium hydroxide
Ammonia solution, also known as ammonium hydroxide, ammonia water, ammonical liquor, ammonia liquor, aqua ammonia, aqueous ammonia, or simply ammonia, is a solution of ammonia in water. It can be denoted by the symbols NH3...
at 900–1000 °C yields oxyfluoride AcOF. Whereas lanthanum oxyfluoride can be easily obtained by burning lanthanum trifluoride in air at 800 °C for an hour, similar treatment of actinium trifluoride yields no AcOF and only results in melting of the initial product.
- AcF3 + 2 NH3 + H2O → AcOF + 2 NH4F
Actinium trichloride is obtained by reacting actinium hydroxide or oxalate
Oxalate
Oxalate , is the dianion with formula C2O42− also written 22−. Either name is often used for derivatives, such as disodium oxalate, 2C2O42−, or an ester of oxalic acid Oxalate (IUPAC: ethanedioate), is the dianion with formula C2O42− also written (COO)22−. Either...
with carbon tetrachloride
Carbon tetrachloride
Carbon tetrachloride, also known by many other names is the organic compound with the formula CCl4. It was formerly widely used in fire extinguishers, as a precursor to refrigerants, and as a cleaning agent...
vapors at temperatures above 960 °C. Similar to oxyfluoride, actinium oxychloride can be prepared by hydrolyzing actinium trichloride with ammonium hydroxide
Ammonium hydroxide
Ammonia solution, also known as ammonium hydroxide, ammonia water, ammonical liquor, ammonia liquor, aqua ammonia, aqueous ammonia, or simply ammonia, is a solution of ammonia in water. It can be denoted by the symbols NH3...
at 1000 °C. However, in contrast to the oxyfluoride, the oxychloride could well be synthesized by igniting a solution of actinium trichloride in hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
with ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
.
Reaction of aluminium bromide
Aluminium bromide
Aluminium bromide is any chemical compound with the empirical formula AlBrx. The species called "aluminium tribromide," is the most common aluminium bromide. The species aluminium monobromide forms from the reaction of HBr with Al metal at high temperature...
and actinium oxide yields actinium tribromide:
- Ac2O3 + 2 AlBr3 → 2 AcBr3 + Al2O3
and treating it with ammonium hydroxide at 500 °C results in the oxybromide AcOBr.
Other compounds
Actinium hydride was obtained by reduction of actinium trichloride with potassium at 300 °C, and its structure was deduced by analogy with the corresponding LaH2 hydride. The source of hydrogen in the reaction was uncertain.Mixing monosodium phosphate
Monosodium phosphate
Monosodium phosphate , also known as anhydrous monobasic sodium phosphate is a chemical compound of sodium with a phosphate counterion. It is used as a laxative and, in combination with other sodium phosphates, as a pH buffer....
(NaH2PO4) with a solution of actinium in hydrochloric acid yields white-colored actinium phosphate hemihydrate (AcPO4·0.5H2O), and heating actinium oxalate with hydrogen sulfide
Hydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
vapors at 1400 °C for a few minutes results in a black actinium sulfide Ac2S3. It may possibly be produced by acting with a mixture of hydrogen sulfide and carbon disulfide on actinium oxide at 1000 °C.
Isotopes
Naturally occurring actinium is composed of one radioactive isotopeIsotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
; . Thirty-six radioisotopes have been identified, the most stable being with a half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of 21.772 years, with a half-life of 10.0 days and with a half-life of 29.37 hours. All remaining radioactive
Radioactive decay
Radioactive decay is the process by which an atomic nucleus of an unstable atom loses energy by emitting ionizing particles . The emission is spontaneous, in that the atom decays without any physical interaction with another particle from outside the atom...
isotopes have half-lives that are less than 10 hours and the majority of them have half-lives shorter than 1 minute. The shortest-lived known isotope of actinium is (half-life of 69 nanoseconds) which decays through alpha decay
Alpha decay
Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle and thereby transforms into an atom with a mass number 4 less and atomic number 2 less...
and electron capture
Electron capture
Electron capture is a process in which a proton-rich nuclide absorbs an inner atomic electron and simultaneously emits a neutrino...
. Actinium also has two meta states.
Purified comes into equilibrium with its decay products at the end of 185 days. It decays according to its 21.773-year half-life emitting mostly beta (98.8%) and some alpha particles (1.2%); the successive decay products are part of the actinium series. Owing to the low available amounts, low energy of its beta particles (46 keV) and low intensity of alpha radiation, is difficult to detect directly by its emission and it is therefore traced via its decay products. The isotopes of actinium range in atomic weight
Atomic weight
Atomic weight is a dimensionless physical quantity, the ratio of the average mass of atoms of an element to 1/12 of the mass of an atom of carbon-12...
from 206 u
Atomic mass unit
The unified atomic mass unit or dalton is a unit that is used for indicating mass on an atomic or molecular scale. It is defined as one twelfth of the rest mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state, and has a value of...
to 236 u .
!Production
!Decay
!Half-life
|-
|221Ac
|232Th(d,9n)225Pa(α)→221Ac
|α
|52 ms
|-
|222Ac
|232Th(d,8n)226Pa(α)→222Ac
|α
|5.0 s
|-
|223Ac
|232Th(d,7n)227Pa(α)→223Ac
|α
|2.1 min
|-
|224Ac
|232Th(d,6n)228Pa(α)→224Ac
|α
|2.78 hours
|-
|225Ac
|232Th(n,γ)233Th(β−)→233Pa(β−)→233U(α)→229Th(α)→225Ra(β−)225Ac
|α
|10 days
|-
|226Ac
|226Ra(d,2n)226Ac
|α, β−
electron capture
|29.37 hours
|-
|227Ac
|235U(α)→231Th(β−)→231Pa(α)→227Ac
|α, β−
|21.77 years
|-
|228Ac
|232Th(α)→228Ra(β−)→228Ac
|β−
|6.15 hours
|-
|229Ac
|228Ra(n,γ)229Ra(β−)→229Ac
|β−
|62.7 min
|-
|230Ac
|232Th(d,α)230Ac
|β−
|122 s
|-
|231Ac
|232Th(γ,p)231Ac
|β−
|7.5 min
|-
|232Ac
|232Th(n,p)232Ac
|β−
|119 s
|}>
Occurrence and synthesis
Actinium is found only in traces in uraniumUranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
ores as 227Ac – one tonne of ore contains about 0.2 milligrams of actinium. These uranium ores sometimes contain lanthanum
Lanthanum
Lanthanum is a chemical element with the symbol La and atomic number 57.Lanthanum is a silvery white metallic element that belongs to group 3 of the periodic table and is the first element of the lanthanide series. It is found in some rare-earth minerals, usually in combination with cerium and...
and other lanthanide
Lanthanide
The lanthanide or lanthanoid series comprises the fifteen metallic chemical elements with atomic numbers 57 through 71, from lanthanum through lutetium...
s. The actinium isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
227Ac is a transient member of the actinium series decay chain
Decay chain
In nuclear science, the decay chain refers to the radioactive decay of different discrete radioactive decay products as a chained series of transformations...
, which begins with the parent isotope 235U
Uranium-235
- References :* .* DOE Fundamentals handbook: Nuclear Physics and Reactor theory , .* A piece of U-235 the size of a grain of rice can produce energy equal to that contained in three tons of coal or fourteen barrels of oil. -External links:* * * one of the earliest articles on U-235 for the...
(or 239Pu
Plutonium-239
Plutonium-239 is an isotope of plutonium. Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons, although uranium-235 has also been used and is currently the secondary isotope. Plutonium-239 is also one of the three main isotopes demonstrated usable as fuel in...
) and ends with the stable lead isotope 207Pb
Isotopes of lead
Lead has four stable isotopes: 204Pb, 206Pb, 207Pb, 208Pb. Lead-204 is entirely a primordial nuclide and is not a radiogenic nuclide. The three isotopes lead-206, lead-207, and lead-208 represent the ends of three decay chains called the uranium series , the actinium series, and the thorium...
. Another actinium isotope (225Ac) is transiently present in the neptunium series decay chain
Decay chain
In nuclear science, the decay chain refers to the radioactive decay of different discrete radioactive decay products as a chained series of transformations...
, beginning with 237Np
Neptunium
Neptunium is a chemical element with the symbol Np and atomic number 93. A radioactive metal, neptunium is the first transuranic element and belongs to the actinide series. Its most stable isotope, 237Np, is a by-product of nuclear reactors and plutonium production and it can be used as a...
(or 233U)
Uranium-235
- References :* .* DOE Fundamentals handbook: Nuclear Physics and Reactor theory , .* A piece of U-235 the size of a grain of rice can produce energy equal to that contained in three tons of coal or fourteen barrels of oil. -External links:* * * one of the earliest articles on U-235 for the...
and ending with near-stable bismuth
Bismuth
Bismuth is a chemical element with symbol Bi and atomic number 83. Bismuth, a trivalent poor metal, chemically resembles arsenic and antimony. Elemental bismuth may occur naturally uncombined, although its sulfide and oxide form important commercial ores. The free element is 86% as dense as lead...
(209Bi).
The low natural concentration, and the close similarity of physical and chemical properties to those of lanthanum and other lanthanides, which are always abundant in actinium-bearing ores, render separation of actinium from the ore impractical, and complete separation was never achieved. Instead, actinium is prepared, in milligram amounts, by the neutron irradiation of 226 in a nuclear reactor
Nuclear reactor
A nuclear reactor is a device to initiate and control a sustained nuclear chain reaction. Most commonly they are used for generating electricity and for the propulsion of ships. Usually heat from nuclear fission is passed to a working fluid , which runs through turbines that power either ship's...
.

The reaction yield is about 2% of the radium weight. 227Ac can further capture neutrons resulting in small amounts of 228Ac. After the synthesis, actinium is separated from radium and from the products of decay and nuclear fusion, such as thorium, polonium, lead and bismuth. The extraction can be performed with thenoyltrifluoroacetone-benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
solution from an aqueous solution of the radiation products, and the selectivity to a certain element is achieved by adjusting the pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
(to about 6.0 for actinium). An alternative procedure is anion exchange with an appropriate resin
Resin
Resin in the most specific use of the term is a hydrocarbon secretion of many plants, particularly coniferous trees. Resins are valued for their chemical properties and associated uses, such as the production of varnishes, adhesives, and food glazing agents; as an important source of raw materials...
in nitric acid
Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
, which can result in a separation factor of 1,000,000 for radium and actinium vs. thorium in a two-stage process. Actinium can then be separated from radium, with a ratio of about 100, using a low cross-linking cation exchange resin and nitric acid as eluant.
225Ac was first produced artificially at the Institute for Transuranium Elements
Institute for Transuranium Elements
The Institute for Transuranium Elements is a European Commission nuclear research institute in Karlsruhe, Germany. The ITU is one of the seven institutes of the Joint Research Centre , a Directorate-General of the European Commission . The ITU has about 300 staff...
(ITU) in Germany using a cyclotron
Cyclotron
In technology, a cyclotron is a type of particle accelerator. In physics, the cyclotron frequency or gyrofrequency is the frequency of a charged particle moving perpendicularly to the direction of a uniform magnetic field, i.e. a magnetic field of constant magnitude and direction...
and at St George Hospital
St George Hospital, Sydney
The St George Hospital and Community Health Service is a tertiary referral hospital located in Kogarah, a southern suburb of Sydney, NSW, Australia...
in Sydney using a linac
Linear particle accelerator
A linear particle accelerator is a type of particle accelerator that greatly increases the velocity of charged subatomic particles or ions by subjecting the charged particles to a series of oscillating electric potentials along a linear beamline; this method of particle acceleration was invented...
in 2000. This rare isotope has potential applications in radiation therapy and is most efficiently produced by bombarding a radium-226 target with 20–30 MeV deuterium
Deuterium
Deuterium, also called heavy hydrogen, is one of two stable isotopes of hydrogen. It has a natural abundance in Earth's oceans of about one atom in of hydrogen . Deuterium accounts for approximately 0.0156% of all naturally occurring hydrogen in Earth's oceans, while the most common isotope ...
ions. This reaction also yields 226Ac which however decays with a half-life of 29 hours and thus does not contaminate 225Ac.
Actinium metal has been prepared by the reduction of actinium fluoride with lithium
Lithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
vapor in vacuum at a temperature between 1100 and 1300 °C. Higher temperatures resulted in evaporation of the product and lower ones lead to an incomplete transformation. Lithium was chosen among other alkali metals because its fluoride is most volatile.
Applications
Owing to its scarcity, high price and radioactivity, actinium currently has no significant industrial use.227Ac is highly radioactive and was therefore studied for use as an active element of radioisotope thermoelectric generator
Radioisotope thermoelectric generator
A radioisotope thermoelectric generator is an electrical generator that obtains its power from radioactive decay. In such a device, the heat released by the decay of a suitable radioactive material is converted into electricity by the Seebeck effect using an array of thermocouples.RTGs can be...
s, for example in spacecraft. The oxide of 227Ac pressed with beryllium
Beryllium
Beryllium is the chemical element with the symbol Be and atomic number 4. It is a divalent element which occurs naturally only in combination with other elements in minerals. Notable gemstones which contain beryllium include beryl and chrysoberyl...
is also an efficient neutron source
Neutron source
A Neutron source is a device that emits neutrons. There is a wide variety of different sources, ranging from hand-held radioactive sources to neutron research facilities operating research reactors and spallation sources...
with the activity exceeding that of the standard americium-beryllium and radium-beryllium pairs. In all those applications, 227Ac (a beta source) is merely a progenitor which generates alpha-emitting isotopes upon its decay. Beryllium captures alpha particles and emits neutrons owing to its large cross-section for the (α,n) nuclear reaction:
The 227AcBe neutron sources can be applied in a neutron probe
Neutron probe
A neutron probe is a device used to measure the quantity of water present in soil.A typical neutron probe contains a pellet of americium-241 and beryllium. The alpha particles emitted by the decay of the americium collide with the light beryllium nuclei, producing fast neutrons...
– a standard device for measuring the quantity of water present in soil, as well as moisture/density for quality control in highway construction. Such probes are also used in well logging applications, in neutron radiography
Neutron Radiography
Neutron Radiography is the process by which film is exposed by first passing neutrons through an object to produce a visible image of the materials that make up the object. Primarily used in scientific investigations.- Brief History of Neutron Imaging :...
, tomography and other radiochemical investigations.

Radiation therapy
Radiation therapy , radiation oncology, or radiotherapy , sometimes abbreviated to XRT or DXT, is the medical use of ionizing radiation, generally as part of cancer treatment to control malignant cells.Radiation therapy is commonly applied to the cancerous tumor because of its ability to control...
, in particular targeted alpha therapy (TAT). This isotope has a half-life of 10 days that makes it much more suitable for radiation therapy than 213Bi (half-life 46 minutes). Not only 225Ac itself, but also its decay products emit alpha particles which kill cancer cells in the body. The major difficulty with application of 225Ac was that intravenous injection of simple actinium complexes resulted in their accumulation in the bones and liver for a period of tens of years. As a result, after the cancer cells were quickly killed by alpha particles from 225Ac, the radiation from the actinium and its decay products might induce new mutations. To solve this problem, 225Ac was bound to a chelating
Chelation
Chelation is the formation or presence of two or more separate coordinate bonds between apolydentate ligand and a single central atom....
agent, such as citrate
Citrate
A citrate can refer either to the conjugate base of citric acid, , or to the esters of citric acid. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate.-Other citric acid ions:...
, ethylenediaminetetraacetic acid (EDTA) or diethylene triamine pentaacetic acid (DTPA). This reduced actinium accumulation in the bones, but the excretion from the body remained slow. Much better results were obtained with such chelating agents as HEHA or DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) coupled to trastuzumab
Trastuzumab
Trastuzumab is a monoclonal antibody that interferes with the HER2/neu receptor.The HER receptors are proteins that are embedded in the cell membrane and communicate molecular signals from outside the cell to inside the cell, and turn genes on and off...
, a monoclonal antibody that interferes with the HER2/neu
HER2/neu
HER-2 also known as proto-oncogene Neu, receptor tyrosine-protein kinase erbB-2, CD340 or p185 is an enzyme that in humans is encoded by the ERBB2 gene. Over expression of this gene is correlated with higher aggressiveness in breast cancers...
receptor
Receptor (biochemistry)
In biochemistry, a receptor is a molecule found on the surface of a cell, which receives specific chemical signals from neighbouring cells or the wider environment within an organism...
. The latter delivery combination was tested on mice and proved to be effective against leukemia
Leukemia
Leukemia or leukaemia is a type of cancer of the blood or bone marrow characterized by an abnormal increase of immature white blood cells called "blasts". Leukemia is a broad term covering a spectrum of diseases...
, lymphoma
Lymphoma
Lymphoma is a cancer in the lymphatic cells of the immune system. Typically, lymphomas present as a solid tumor of lymphoid cells. Treatment might involve chemotherapy and in some cases radiotherapy and/or bone marrow transplantation, and can be curable depending on the histology, type, and stage...
, breast
Breast cancer
Breast cancer is cancer originating from breast tissue, most commonly from the inner lining of milk ducts or the lobules that supply the ducts with milk. Cancers originating from ducts are known as ductal carcinomas; those originating from lobules are known as lobular carcinomas...
, ovarian
Ovarian cancer
Ovarian cancer is a cancerous growth arising from the ovary. Symptoms are frequently very subtle early on and may include: bloating, pelvic pain, difficulty eating and frequent urination, and are easily confused with other illnesses....
, neuroblastoma
Neuroblastoma
Neuroblastoma is the most common extracranial solid cancer in childhood and the most common cancer in infancy, with an annual incidence of about 650 cases per year in the US , and 100 cases per year in the UK . Close to 50 percent of neuroblastoma cases occur in children younger than two years old...
and prostate cancer
Prostate cancer
Prostate cancer is a form of cancer that develops in the prostate, a gland in the male reproductive system. Most prostate cancers are slow growing; however, there are cases of aggressive prostate cancers. The cancer cells may metastasize from the prostate to other parts of the body, particularly...
s.
The medium half-life of 227Ac (21.77 years) makes it very convenient radioactive isotope in modeling the slow vertical mixing of oceanic waters. The associated processes cannot be studied with the required accuracy by direct measurements of current velocities (of the order 50 meters per year). However, evaluation of the concentration depth-profiles for different isotopes allows estimating the mixing rates. The physics behind this method is as follows: oceanic waters contain homogeneously dispersed 235U. Its decay product, 231Pa, gradually precipitates to the bottom, so that its concentration first increases with depth and then stays nearly constant. 231Pa decays to 227Ac; however, the concentration of the latter isotope does not follow the 231Pa depth profile, but instead increases toward the sea bottom. This occurs because of the mixing processes which raise some additional 227Ac from the sea bottom. Thus analysis of both 231Pa and 227Ac depth profiles allows to model the mixing behavior.
Precautions
227Ac is highly radioactive and experiments with it are carried out in a specially designed laboratory equipped with a glove box and radiation shielding. When actinium trichloride is administered intravenously to rats, about 33% of actinium is deposited into the bones and 50% into the liver. Its toxicity is comparable, but slightly lower than that of americium and plutonium.See also
- Actinium series