
Acids in wine
Encyclopedia
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
s in wine
Wine
Wine is an alcoholic beverage, made of fermented fruit juice, usually from grapes. The natural chemical balance of grapes lets them ferment without the addition of sugars, acids, enzymes, or other nutrients. Grape wine is produced by fermenting crushed grapes using various types of yeast. Yeast...
are an important component in both winemaking
Winemaking
Winemaking, or vinification, is the production of wine, starting with selection of the grapes or other produce and ending with bottling the finished wine. Although most wine is made from grapes, it may also be made from other fruit or non-toxic plant material...
and the finished product of wine. They are present in both grape
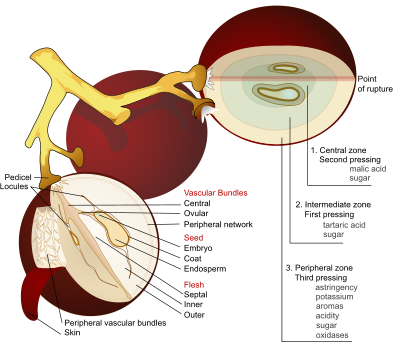
Grape
A grape is a non-climacteric fruit, specifically a berry, that grows on the perennial and deciduous woody vines of the genus Vitis. Grapes can be eaten raw or they can be used for making jam, juice, jelly, vinegar, wine, grape seed extracts, raisins, molasses and grape seed oil. Grapes are also...
s and wine, having direct influences on the color, balance and taste of the wine as well as the growth and vitality of yeast
Yeast
Yeasts are eukaryotic micro-organisms classified in the kingdom Fungi, with 1,500 species currently described estimated to be only 1% of all fungal species. Most reproduce asexually by mitosis, and many do so by an asymmetric division process called budding...
s during fermentation
Fermentation (wine)
The process of fermentation in wine turns grape juice into an alcoholic beverage. During fermentation, yeast interact with sugars in the juice to create ethanol, commonly known as ethyl alcohol, and carbon dioxide...
and protecting the wine from bacteria
Bacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
. The measure of the amount of acidity in wine is known as the “Titratable Acidity” or “Total acidity”, which refers to the test that yields the total of all acids present, while strength of acidity is measured according to pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
with most wines having a pH between 2.9–3.9. Generally, the lower the pH, the higher the acidity in the wine. However, there is no direct connection between total acidity and pH (it is possible to find wines with a high pH for wine and high acidity). In wine tasting
Wine tasting
Wine tasting is the sensory examination and evaluation of wine. While the practice of wine tasting is as ancient as its production, a more formalized methodology has slowly become established from the 14th century onwards...
, the term “acidity” refers to the fresh, tart and sour attributes of the wine which is evaluated in relation to how well the acidity balances out the sweetness and bitter components of the wine such as tannins. There are three primary acids found in wine grapes: tartaric, malic and citric. During the course of winemaking and in the finished wines, acetic
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
, butyric
Butyric acid
Butyric acid , also known under the systematic name butanoic acid, is a carboxylic acid with the structural formula CH3CH2CH2-COOH. Salts and esters of butyric acid are known as butyrates or butanoates...
, lactic and succinic acid
Succinic acid
Succinic acid is a dicarboxylic acid. Succinate plays a biochemical role in the citric acid cycle. The name derives from Latin succinum, meaning amber, from which the acid may be obtained....
can play significant roles. Most of the acids involved with wine are fixed acids with the notable exception of acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
, mostly found in vinegar, which is volatile and can contribute to the wine fault
Wine fault
A wine fault or defect is an unpleasant characteristic of a wine often resulting from poor winemaking practices or storage conditions, and leading to wine spoilage. Many of the compounds that cause wine faults are already naturally present in wine but at insufficient concentrations to adversely...
known as volatile acidity. Sometimes additional acids are used in winemaking such as ascorbic, sorbic and sulfurous acid
Sulfurous acid
Sulfurous acid is the chemical compound with the formula H2SO3. There is no evidence that sulfurous acid exists in solution, but the molecule has been detected in the gas phase...
s.
Tartaric acid
- Also see general article tartaric acidTartaric acidTartaric acid is a white crystalline diprotic organic acid. It occurs naturally in many plants, particularly grapes, bananas, and tamarinds; is commonly combined with baking soda to function as a leavening agent in recipes, and is one of the main acids found in wine. It is added to other foods to...
.

Organic acid
An organic acid is an organic compound with acidic properties. The most common organic acids are the carboxylic acids, whose acidity is associated with their carboxyl group –COOH. Sulfonic acids, containing the group –SO2OH, are relatively stronger acids. The relative stability of the conjugate...
is rare but it is found in significant concentrations in grape vines. Along with malic acid, and to a lesser extent citric acid, tartaric is one of the fixed acids found in wine grapes. The concentration varies depending on grape variety and the soil content of the vineyard. Some varieties, such as Palomino
Palomino (grape)
Palomino is a white grape widely grown in Spain and South Africa, and best known for its use in the manufacture of sherry.-Wine regions:In Spain, the grape is split into the sub-varieties Palomino Fino, Palomino Basto, and Palomino de Jerez, of which Palomino Fino is by far the most important,...
, are naturally deposed to having high levels of tartaric acids while Malbec
Malbec
Malbec is a purple grape variety used in making red wine. The grapes tend to have an inky dark color and robust tannins, and are long known as one of the six grapes allowed in the blend of red Bordeaux wine. The French plantations of Malbec are now found primarily in Cahors in the South West...
and Pinot noir
Pinot Noir
Pinot noir is a black wine grape variety of the species Vitis vinifera. The name may also refer to wines created predominantly from Pinot noir grapes...
generally have lower levels. During flowering, there are high levels of tartaric acid concentrated in the grape flowers and then young berries. As the vine progresses through ripening, tartaric does not get metabolized through respiration like malic acid so that the level of tartaric acid in the grape vines remains relatively consistent throughout the ripening process.
Less than half of the tartaric acid found in grapes is free standing, with the majority of the concentration present as potassium acid salt. During fermentation, these tartrate
Tartrate
A tartrate is a salt or ester of the organic compound tartaric acid, a dicarboxylic acid. Its formula is O−OC-CH-CH-COO− or C4H4O62−.As food additives, tartrates are used as antioxidants, acidity regulators, and emulsifiers...
s bind with the lees, pulp debris and precipitated tannins and pigments. While there is some variance among grape varieties and wine regions, generally about half of the deposits are soluble in the alcoholic mixture of wine. The crystallization of these tartrates can happen at unpredictable times and in a wine bottle appear like broken glass though they are in fact harmless. Winemakers will often put the wine through cold stabilization where it is exposed temperatures below freezing to encourage the tartrates to crystallize and precipitate out of the wine.
Malic acid
- Also see general article malic acidMalic acidMalic acid is an organic compound with the formula HO2CCH2CHOHCO2H. It is a dicarboxylic acid which is made by all living organisms, contributes to the pleasantly sour taste of fruits, and is used as a food additive. Malic acid has two stereoisomeric forms , though only the L-isomer exists...
.

Fruit
In broad terms, a fruit is a structure of a plant that contains its seeds.The term has different meanings dependent on context. In non-technical usage, such as food preparation, fruit normally means the fleshy seed-associated structures of certain plants that are sweet and edible in the raw state,...
and berry
Berry
The botanical definition of a berry is a fleshy fruit produced from a single ovary. Grapes are an example. The berry is the most common type of fleshy fruit in which the entire ovary wall ripens into an edible pericarp. They may have one or more carpels with a thin covering and fleshy interiors....
plant but it's most often associated with green apples from which flavor it most readily projects in wine. Its name comes from the Latin malum meaning “apple”. In the grape vine, malic acid is involved in several processes which are essential for the health and sustainability of the vine. Its chemical structure allows it to participate in enzymatic reactions that transport energy throughout the vine. The concentration of malic acids varies depending on the grape variety with some varieties, like Barbera
Barbera
Barbera is a red Italian wine grape variety that, as of 2000, was the third most-planted red grape variety in Italy . It produces good yields and is known for deep color, low tannins and high levels of acid...
, Carignan and Sylvaner being naturally deposed to high levels. The levels of malic acid in grape berries are at their peak just before veraison
Veraison
Véraison is a viticulture term meaning "the onset of ripening". It is originally French, but has been adopted into English use...
when they can be found in concentrations as high as 20 g
Gram
The gram is a metric system unit of mass....
/L
Litre
pic|200px|right|thumb|One litre is equivalent to this cubeEach side is 10 cm1 litre water = 1 kilogram water The litre is a metric system unit of volume equal to 1 cubic decimetre , to 1,000 cubic centimetres , and to 1/1,000 cubic metre...
. As the vine progresses through the ripening stage, malic acid is metabolized in the process of respiration and by harvest
Harvest (wine)
The harvesting of wine grapes is one of the most crucial steps in the process of winemaking. The time of harvest is determined primarily by the ripeness of the grape as measured by sugar, acid and tannin levels with winemakers basing their decision to pick based on the style of wine they wish to...
its concentration could be as low as 1 to 9 g/L. The respiratory loss of malic acid is more pronounced in warmer climates. When all the malic acid is used up in the grape it is considered “over-ripe” or senescent. Winemakers must compensate for this loss by manually adding acid at the winery in a process known as acidification.
Malic acid can be further reduced during the winemaking process through malolactic fermentation
Malolactic fermentation
Malolactic fermentation is a process in winemaking where tart-tasting malic acid, naturally present in grape must, is converted to softer-tasting lactic acid. Malolactic fermentation tends to create a rounder, fuller mouthfeel. It has been said that malic acid tastes of green apples...
or MLF. In this process bacteria convert the stronger malic acid into the softer lactic acid: formally, malic acid is polyprotic (contributes multiple protons, here 2), while lactic acid is monoprotic (contributes 1 proton), and thus has only half the effect on acidity (pH); also, the first acidity constant
Acid dissociation constant
An acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
(pKa) of malic acid (3.4 at room temperature) is lower than the (single) acidity constant of lactic acid (3.86 at room temperature), indicating stronger acidity. Thus after MLF, wine has higher pH (less acidic), and different mouthfeel.
The bacteria behind this process can be found naturally in the winery, in cooperages which make oak wine barrels that will house a population of the bacteria or they can be manually introduced by the winemaker with a cultured specimen. For some wines, the conversion of malic into lactic acid can be beneficial, especially if the wine has excessive levels of malic acid. For other wines, such as Chenin blanc
Chenin Blanc
Chenin blanc , is a white wine grape variety from the Loire valley of France. Its high acidity means it can be used to make everything from sparkling wines to well-balanced dessert wines, although it can produce very bland, neutral wines if the vine's natural vigor is not controlled...
and Riesling
Riesling
Riesling is a white grape variety which originated in the Rhine region of Germany. Riesling is an aromatic grape variety displaying flowery, almost perfumed, aromas as well as high acidity. It is used to make dry, semi-sweet, sweet and sparkling white wines. Riesling wines are usually varietally...
, it produces off flavors in the wine (such as the butter
Butter
Butter is a dairy product made by churning fresh or fermented cream or milk. It is generally used as a spread and a condiment, as well as in cooking applications, such as baking, sauce making, and pan frying...
y smell of diacetyl
Diacetyl
Diacetyl is a natural byproduct of fermentation. It is a vicinal diketone with the molecular formula C4H6O2...
) that would not be appealing for that variety. In general, red wines are more often put through MLF than whites, which means that there is a higher likelihood of finding malic acid in white wines (though there are notable exceptions like oaked Chardonnay
Chardonnay
Chardonnay is a green-skinned grape variety used to make white wine. It is originated from the Burgundy wine region of eastern France but is now grown wherever wine is produced, from England to New Zealand...
which is often put through MLF).
Lactic acid
- Also see general article lactic acidLactic acidLactic acid, also known as milk acid, is a chemical compound that plays a role in various biochemical processes and was first isolated in 1780 by the Swedish chemist Carl Wilhelm Scheele. Lactic acid is a carboxylic acid with the chemical formula C3H6O3...
.

Milk
Milk is a white liquid produced by the mammary glands of mammals. It is the primary source of nutrition for young mammals before they are able to digest other types of food. Early-lactation milk contains colostrum, which carries the mother's antibodies to the baby and can reduce the risk of many...
y” flavors in wine and is the primary acid of yogurt and sauerkraut
Sauerkraut
Sauerkraut , directly translated from German: "sour cabbage", is finely shredded cabbage that has been fermented by various lactic acid bacteria, including Leuconostoc, Lactobacillus, and Pediococcus. It has a long shelf-life and a distinctive sour flavor, both of which result from the lactic acid...
. It is produced during winemaking by lactic acid bacteria (known as LAB) which includes three genera
Genera
Genera is a commercial operating system and development environment for Lisp machines developed by Symbolics. It is essentially a fork of an earlier operating system originating on the MIT AI Lab's Lisp machines which Symbolics had used in common with LMI and Texas Instruments...
: Oenococcus
Oenococcus
Oenococcus is a genus of Gram-positive bacteria, placed within the family of Leuconostocaceae. The only species in the genus used to be Oenococcus oeni . In 2006, the species Oenococcus kitaharae was identified...
, Pediococcus
Pediococcus
Pediococcus is a genus of Gram-positive lactic acid bacteria, placed within the family of Lactobacillaceae. They usually occur in pairs or tetrads, and divide along two planes of symmetry, as do the other lactic acid cocci genera Aerococcus and Tetragenococcus. They are purely homofermentative...
and Lactobacillus
Lactobacillus
Lactobacillus is a genus of Gram-positive facultative anaerobic or microaerophilic rod-shaped bacteria. They are a major part of the lactic acid bacteria group, named as such because most of its members convert lactose and other sugars to lactic acid. They are common and usually benign...
. These bacterium convert both sugar and malic acid into lactic acid, the latter through a process known as malolactic fermentation
Malolactic fermentation
Malolactic fermentation is a process in winemaking where tart-tasting malic acid, naturally present in grape must, is converted to softer-tasting lactic acid. Malolactic fermentation tends to create a rounder, fuller mouthfeel. It has been said that malic acid tastes of green apples...
(MLF). The process of converting malic into lactic acid can be beneficial for some wines, adding complexity and softening the harshness of malic acidity but it can generate off flavors and turbidity
Turbidity
Turbidity is the cloudiness or haziness of a fluid caused by individual particles that are generally invisible to the naked eye, similar to smoke in air. The measurement of turbidity is a key test of water quality....
in others. Some strains of LAB can produce biogenic amines like histamine
Histamine
Histamine is an organic nitrogen compound involved in local immune responses as well as regulating physiological function in the gut and acting as a neurotransmitter. Histamine triggers the inflammatory response. As part of an immune response to foreign pathogens, histamine is produced by...
, tyramine
Tyramine
Tyramine is a naturally occurring monoamine compound and trace amine derived from the amino acid tyrosine. Tyramine acts as a catecholamine releasing agent...
and putrescine
Putrescine
Putrescine is a foul-smelling organic chemical compound NH24NH2 that is related to cadaverine; both are produced by the breakdown of amino acids in living and dead organisms and both are toxic in large doses...
which may be a cause of red wine headache
Red wine headache
Red wine headache is a headache often accompanied by nausea and flushing that occurs in many people after drinking even a single glass of red wine. This syndrome can sometimes develop within 15 minutes of consumption of the wine....
s in some wine drinkers. Winemakers wishing to control or prevent MLF can use sulfur dioxide
Sulfur dioxide
Sulfur dioxide is the chemical compound with the formula . It is released by volcanoes and in various industrial processes. Since coal and petroleum often contain sulfur compounds, their combustion generates sulfur dioxide unless the sulfur compounds are removed before burning the fuel...
to stun the bacteria. Racking the wine quickly off its lees will also help control the bacteria since lees are a vital food source for them. They must also be very careful of what wine barrels and winemaking equipment that the wine is exposed to because of the bacteria's ability to deeply embed themselves within wood fibers. A wine barrel that has completed one successful malolactic fermentation will almost always induce MLF in every wine that gets stored in it from then on.
Citric acid
- Also see general article citric acidCitric acidCitric acid is a weak organic acid. It is a natural preservative/conservative and is also used to add an acidic, or sour, taste to foods and soft drinks...
.
While very common in citrus fruits, such as limes
Lime (fruit)
Lime is a term referring to a number of different citrus fruits, both species and hybrids, which are typically round, green to yellow in color, 3–6 cm in diameter, and containing sour and acidic pulp. Limes are a good source of vitamin C. Limes are often used to accent the flavors of foods and...
, citric acid is found only in very minute quantities in wine grapes. It often has a concentration about 1/20 that of tartaric acid. The citric acid most commonly found in wine is commercially produced acid supplements derived from fermenting
Fermentation (food)
Fermentation in food processing typically is the conversion of carbohydrates to alcohols and carbon dioxide or organic acids using yeasts, bacteria, or a combination thereof, under anaerobic conditions. Fermentation in simple terms is the chemical conversion of sugars into ethanol...
sucrose
Sucrose
Sucrose is the organic compound commonly known as table sugar and sometimes called saccharose. A white, odorless, crystalline powder with a sweet taste, it is best known for its role in human nutrition. The molecule is a disaccharide composed of glucose and fructose with the molecular formula...
solutions. These inexpensive supplements can be used by winemakers in acidification to boost the total acidity of the wine. It is used less frequently than tartaric and malic due to aggressive citric flavors that it can add to the wine. When citric acid is added, it is always done after primary alcohol fermentation has been completed due to the tendency of yeast to convert citric into acetic acid. In the European Union
European Union
The European Union is an economic and political union of 27 independent member states which are located primarily in Europe. The EU traces its origins from the European Coal and Steel Community and the European Economic Community , formed by six countries in 1958...
, use of citric acid for acidification is prohibited but limited use of citric acid is permitted for removing excess iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
and copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
from the wine if potassium ferrocyanide
Potassium ferrocyanide
Potassium ferrocyanide is the inorganic compound with formula K4[Fe6]•3H2O. It is the potassium salt of the coordination complex [Fe6]4-. This salt forms lemon-yellow monoclinic crystals.-Synthesis:...
is not available.
Other acids
Acetic acidAcetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
is a two-carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
organic acid produced in wine during or after the fermentation period. It is the most volatile of the primary acids associated with wine and is responsible for the sour taste of vinegar
Vinegar
Vinegar is a liquid substance consisting mainly of acetic acid and water, the acetic acid being produced through the fermentation of ethanol by acetic acid bacteria. Commercial vinegar is produced either by fast or slow fermentation processes. Slow methods generally are used with traditional...
. During fermentation, activity by yeast cells naturally produces a small amount of acetic acid. If the wine is exposed to oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
, acetobacter bacteria will convert the ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
into acetic acid. This process is known as the “acetification” of wine and is the primary process behind wine degradation into vinegar. An excessive amount of acetic acid is also considered a wine fault
Wine fault
A wine fault or defect is an unpleasant characteristic of a wine often resulting from poor winemaking practices or storage conditions, and leading to wine spoilage. Many of the compounds that cause wine faults are already naturally present in wine but at insufficient concentrations to adversely...
. A taster's sensitivity to acetic acid will vary, but most people can detect excessive amounts at around 600 mg/L.
Ascorbic acid
Ascorbic acid
Ascorbic acid is a naturally occurring organic compound with antioxidant properties. It is a white solid, but impure samples can appear yellowish. It dissolves well in water to give mildly acidic solutions. Ascorbic acid is one form of vitamin C. The name is derived from a- and scorbutus , the...
, also known as vitamin C
Vitamin C
Vitamin C or L-ascorbic acid or L-ascorbate is an essential nutrient for humans and certain other animal species. In living organisms ascorbate acts as an antioxidant by protecting the body against oxidative stress...
, is found in young wine grapes prior to veraison but is rapidly lost throughout the ripening process. In winemaking it is used with sulfur dioxide as an anti-oxidant to prevent oxidation, often added during the bottling process for white wines. In the European Union, use of ascorbic acid as an additive is limited to 150 mg/L.
Butyric acid
Butyric acid
Butyric acid , also known under the systematic name butanoic acid, is a carboxylic acid with the structural formula CH3CH2CH2-COOH. Salts and esters of butyric acid are known as butyrates or butanoates...
is a bacteria-induced wine fault that can cause a wine to smell of spoiled Camembert
Camembert
Camembert is a commune in the Orne department in north-western France.It is most famous as the place where camembert cheese originated.Camembert has been called "The largest small village in France." This is because the area of the commune itself is out of proportion to the center of the village...
or rancid butter.
Sorbic acid
Sorbic acid
Sorbic acid, or 2,4-hexadienoic acid, is a natural organic compound used as a food preservative. It has the chemical formula C6H8O2. It is a colourless solid that is slightly soluble in water and sublimes readily...
is a winemaking additive used often in sweet wines as a preservative against fungi, bacteria and yeast growth. Unlike sulfur dioxide, it does not hinder the growth of the lactic acid bacteria. In the European Union there is a limitation on the amount of sorbic acid that can be added — no more than 200 mg/L. Most humans have a detection threshold of 135 mg/L, with some having a sensitivity to detect its presence at 50 mg/L. Sorbic acid can produce off-flavors and aromas which can be described as “rancid”. When lactic acid bacteria metabolizes sorbates in the wine, it creates a wine fault that is most recognizable by an aroma of crushed Pelargonium
Pelargonium
Pelargonium is a genus of flowering plants which includes about 200 species of perennials, succulents, and shrubs, commonly known as scented geraniums or storksbills. Confusingly, Geranium is the correct botanical name of a separate genus of related plants often called Cranesbills. Both Geranium...
geranium leaves.
Succinic acid
Succinic acid
Succinic acid is a dicarboxylic acid. Succinate plays a biochemical role in the citric acid cycle. The name derives from Latin succinum, meaning amber, from which the acid may be obtained....
is most commonly found in wine but can also be present in trace amounts in ripened grapes. While concentration varies among grape varieties, it is usually found in higher levels with red wine grapes. The acid is created as a by-product
By-product
A by-product is a secondary product derived from a manufacturing process or chemical reaction. It is not the primary product or service being produced.A by-product can be useful and marketable or it can be considered waste....
of the metabolization of nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
by yeast cells during fermentation. The combination of succinic acid with one molecule of ethanol will create the ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
mono-ethyl succinate that is responsible for a mild, fruit aroma in wines.
In winemaking

Veraison
Véraison is a viticulture term meaning "the onset of ripening". It is originally French, but has been adopted into English use...
, which ushers in the ripening period of the annual cycle of grape vines. As the grapes ripen, their sugar level increases and their acidity decreases. Through the process of respiration
Cellular respiration
Cellular respiration is the set of the metabolic reactions and processes that take place in the cells of organisms to convert biochemical energy from nutrients into adenosine triphosphate , and then release waste products. The reactions involved in respiration are catabolic reactions that involve...
, malic acid is metabolized by the grape vine. Grapes from cooler climate wine regions generally have a higher level of acidity due to the slower ripening process which is accelerated by warmer temperatures. The level of acidity still present in the grape is an important consideration for winemakers in deciding when to begin harvest
Harvest (wine)
The harvesting of wine grapes is one of the most crucial steps in the process of winemaking. The time of harvest is determined primarily by the ripeness of the grape as measured by sugar, acid and tannin levels with winemakers basing their decision to pick based on the style of wine they wish to...
. For wines such as Champagne and other sparklers
Sparkling wine
Sparkling wine is a wine with significant levels of carbon dioxide in it making it fizzy. The carbon dioxide may result from natural fermentation, either in a bottle, as with the méthode champenoise, in a large tank designed to withstand the pressures involved , or as a result of carbon dioxide...
, having high levels of acidity is even more vital to the winemaking process and so grapes are often picked under-ripe and at higher acid levels.
In the winemaking process, acids aid in enhancing the effectiveness of sulfur dioxide
Sulfur dioxide
Sulfur dioxide is the chemical compound with the formula . It is released by volcanoes and in various industrial processes. Since coal and petroleum often contain sulfur compounds, their combustion generates sulfur dioxide unless the sulfur compounds are removed before burning the fuel...
to protect the wines from spoilage and can also protect the wine from bacteria due to the inability of most bacteria to survive in an acidic solution. Two notable exceptions to this are acetobacter
Acetobacter
Acetobacter is a genus of acetic acid bacteria characterized by the ability to convert ethanol to acetic acid in the presence of oxygen. There are several species within this genus, and there are other bacteria capable of forming acetic acid under various conditions; but all of the Acetobacter are...
and the lactic acid bacteria. In red wines, acidity helps preserve and stabilize the color of the wine. The ionization
Ionization
Ionization is the process of converting an atom or molecule into an ion by adding or removing charged particles such as electrons or other ions. This is often confused with dissociation. A substance may dissociate without necessarily producing ions. As an example, the molecules of table sugar...
of anthocyanins is affected by pH so wines with lower pH (such as Sangiovese
Sangiovese
Sangiovese is a red Italian wine grape variety whose name derives from the Latin sanguis Jovis, "the blood of Jove"...
based wines) have redder colors that are more stable. Wines with higher pH (such as Syrah based wines) have more blue pigments that are less stable, eventually taking on a muddy grey hue. These wines can also develop a brownish tinge. In white wines, higher pH (lower acidity) causes the phenolics in the wine
Phenolic compounds in wine
The phenolic compounds - natural phenol and polyphenols - in wine include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenes, flavonols, dihydroflavonols, anthocyanins, flavanol monomers and...
to darken and eventually polymerize as brown deposits.
Winemakers will sometimes add acids to the wine (this is called acidification) to make the wine more acid. Doing this is most common in warm climate regions where grapes are often harvested at advanced stages of ripeness with high levels of sugars but very low levels of acid. Tartaric acid is most often added, but winemakers will sometimes add citric or malic acid. Acids can be added either before or after primary fermentation. They can be added during blending or aging, but the increased acidity will become more noticeable to wine tasters if added at this point.
In wine tasting
The acidity in wine is an important component in the quality and taste of the wine. It adds a sharpness to the flavors and is detected most readily by a prickling sensation on the sides of the tongue and a mouth watering after taste. Of particular importance is the balance of acidity versus the sweetness of the wine (the left over residual sugar) and the more bitter components of the wine (most notably tannins but also includes other phenolicsPhenolic compounds in wine
The phenolic compounds - natural phenol and polyphenols - in wine include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenes, flavonols, dihydroflavonols, anthocyanins, flavanol monomers and...
). A wine with too much acidity will taste excessive sour and sharp. A wine with too little acidity will taste flabby, flat and with less defined flavors.

