
Acid-base titration
Encyclopedia
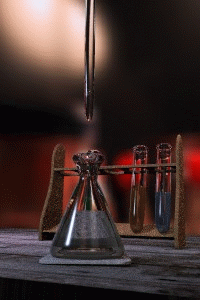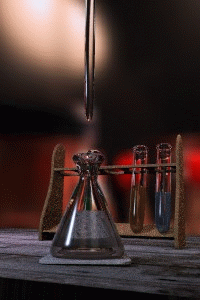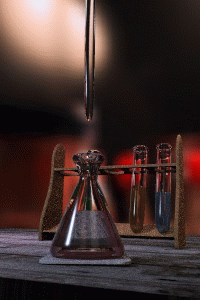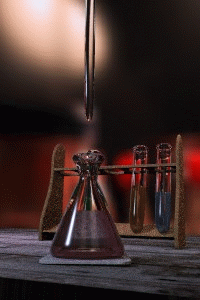
Titration
Titration, also known as titrimetry, is a common laboratory method of quantitative chemical analysis that is used to determine the unknown concentration of an identified analyte. Because volume measurements play a key role in titration, it is also known as volumetric analysis. A reagent, called the...
is the determination of the concentration of an acid or base by exactly neutralizing the acid/base with an acid or base of known concentration. This allows for quantitative analysis
Quantitative analysis (chemistry)
In chemistry, quantitative analysis is the determination of the absolute or relative abundance of one, several or all particular substance present in a sample....
of the concentration of an unknown acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
or base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
. It makes use of the neutralization reaction that occurs between acids and bases and the knowledge of how acids and bases will react if their formulas are known.
Acid–base titrations can also be used to find percent purity of chemicals.
Alkalimetry and acidimetry
Alkalimetry, sometimes spelled alkimetry, is the specialized analytic use of acid-base titration to determine the concentration of a basic (synonymous to alkaline) substance. Acidimetry, sometimes spelled acidometry, is the same concept of specialized analytic acid-base titration, but for an acidic substance.Equipment
The key equipment used in a titration are:- BuretteBuretteA burette is a vertical cylindrical piece of laboratory glassware with a volumetric graduation on its full length and a precision tap, or stopcock, on the bottom. It is used to dispense known amounts of a liquid reagent in experiments for which such precision is necessary, such as a titration...
- White tile - used to see a colour change in the solution
- PipettePipetteA pipette is a laboratory tool used to transport a measured volume of liquid.-Use and variations:Pipettes are commonly used in molecular biology, analytical chemistry as well as medical tests...
- pH indicatorPH indicatorA pH indicator is a halochromic chemical compound that is added in small amounts to a solution so that the pH of the solution can be determined visually. Hence a pH indicator is a chemical detector for hydronium ions or hydrogen ions in the Arrhenius model. Normally, the indicator causes the...
(the one used varies depending on the reactants) - Erlenmeyer flaskErlenmeyer flaskAn Erlenmeyer flask, also known as a conical flask, is a widely used type of laboratory flask which features a flat bottom, a conical body, and a cylindrical neck. It is named after the German chemist Emil Erlenmeyer, who created it in 1861...
/ Conical flask - Titrant or titrator (a standard solutionStandard solutionIn analytical chemistry, a standard solution is a solution containing a precisely known concentration of an element or a substance i.e, a known weight of solute is dissolved to make a specific volume. It is prepared using a standard substance, such as a primary standard. Standard solutions are used...
of known concentration, a common one is aqueous sodium carbonateSodium carbonateSodium carbonate , Na2CO3 is a sodium salt of carbonic acid. It most commonly occurs as a crystalline heptahydrate, which readily effloresces to form a white powder, the monohydrate. Sodium carbonate is domestically well-known for its everyday use as a water softener. It can be extracted from the...
) - AnalyteAnalyteAn analyte, or component , is a substance or chemical constituent that is of interest in an analytical procedure. Grammatically, it is important to note that experiments always seek to measure properties of analytes—and that analytes themselves can never be measured. For instance, one cannot...
or titrand (solution of unknown concentration)
Method
Before starting the titration a suitable pH indicatorPH indicator
A pH indicator is a halochromic chemical compound that is added in small amounts to a solution so that the pH of the solution can be determined visually. Hence a pH indicator is a chemical detector for hydronium ions or hydrogen ions in the Arrhenius model. Normally, the indicator causes the...
must be chosen. The equivalence point of the reaction, the point at which equivalent amounts of the reactants have reacted, will have a pH dependent on the relative strengths of the acid and base used. The pH of the equivalence point can be estimated using the following rules:
- A strong acid will react with a strong base to form a neutral (pH=7) solution.
- A strong acid will react with a weak base to form an acidic (pH<7) solution.
- A weak acid will react with a strong base to form a basic (pH>7) solution.
When a weak acid reacts with a weak base, the equivalence point solution will be basic if the base is stronger and acidic if the acid is stronger. If both are of equal strength, then the equivalence pH will be neutral. However, weak acids are not often titrated against weak bases because the colour change shown with the indicator is often quick, and therefore very difficult for the observer to see the change of colour.
The point at which the indicator changes colour is called the end point. A suitable indicator should be chosen, preferably one that will experience a change in colour (an end point) close to the equivalence point of the reaction.
First, the burette should be rinsed with the standard solution, the pipette with the unknown solution, and the conical flask with distilled water.
Secondly, a known volume of the unknown concentration solution should be taken with the pipette and placed into the conical flask, along with a small amount of the indicator chosen. The burette should always be filled to the top of its scale with the known solution for ease of reading.
The known solution should then be allowed out of the burette, into the conical flask. At this stage we want a rough estimate of the amount of this solution it took to neutralize the unknown solution. The solution should be let out of the burette until the indicator changes colour and the value on the burette should be recorded. This is the first (or rough) titre and should be discluded from any calculations.
Three more titrations should be performed, this time more accurately, taking into account roughly where the end point will occur. The readings on the burette at the end point should be recorded, and averaged to give a final result. The end point is reached when the indicator just changes colour permanently. This is best achieved by washing a hanging drop from the tip of the burette into the flask right at the end of the titration to achieve a drop that is smaller in volume than what can usually be achieved by just dripping solution off the burette.
Acid–base titration is performed with a phenolphthalein
Phenolphthalein
Phenolphthalein is a chemical compound with the formula C20H14O4 and is often written as "HIn" or "phph" in shorthand notation. Often used in titrations, it turns colorless in acidic solutions and pink in basic solutions...
indicator, when it is a strong acid - strong base titration, a bromthymol blue indicator in weak acid - weak base reactions, and a methyl orange
Methyl orange
Methyl orange is a pH indicator frequently used in titrations.It is often used in titrations because of its clear and distinct colour change. Because it changes colour at the pH of a mid-strength acid, it is usually used in titrations for acids...
indicator for strong acid - weak base reactions. If the base is off the scale, i.e. a pH of >13.5, and the acid has a pH >5.5, then an Alizarine yellow indicator may be used. On the other hand, if the acid is off the scale, i.e. a pH of <0.5, and the base has a pH <8.5, then a Thymol Blue
Thymol blue
Thymol blue is a brownish-green or reddish-brown crystalline powder that is used as a pH indicator. It is insoluble in water but soluble in alcohol and dilute alkali solutions. It transitions from red to yellow at pH 1.2–2.8 and from yellow to blue at pH 8.0–9.6.-Bibliography:* Merck. "Thymol...
indicator may be used.
Titration of weak acid
The pH of a weak acidWeak acid
A weak acid is an acid that dissociates incompletely. It does not release all of its hydrogens in a solution, donating only a partial amount of its protons to the solution...
solution being titrated with a strong base solution can be found at different points along the way. These points fall into one of four categories :
- initial pH
- pH before the equivalence point
- pH at the equivalence point
- pH after the equivalence point
1. The initial pH is found for a weak acid
Weak acid
A weak acid is an acid that dissociates incompletely. It does not release all of its hydrogens in a solution, donating only a partial amount of its protons to the solution...
solution in water using the equation

where Ka is the dissociation constant and F is the concentration of the acid.
2. The pH before the equivalence point depends on the amount of weak acid remaining and the amount of conjugate base formed. The pH can be calculated by the following formula (which is a variation of the Henderson-Hasselbalch equation
Henderson-Hasselbalch equation
In chemistry, the Henderson–Hasselbalch equation describes the derivation of pH as a measure of acidity in biological and chemical systems...
):

where:
- pKa is the negative log of the acid dissociation constantAcid dissociation constantAn acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
of the weak acid. - nOH- added is the number of moles of added strong base in the solution.
- nHA initial is the number of moles the weak acid initially present.
When the numerator of the log term equals the denominator (
 ), then the ratio goes to 1 and the log term goes to zero. Thus the pH will equal the pKa which occurs half-way to the equivalence point.
), then the ratio goes to 1 and the log term goes to zero. Thus the pH will equal the pKa which occurs half-way to the equivalence point.3. At the equivalence point, the weak acid is consumed and converted to its weak conjugate base. The pH will be greater than 7 and can be calculated from an equation derived from the following relationships:
- pH + pOH = 14
- KaKb = 10−14
- at equivalence CaVa = CbVb
The previous 3 relationships are used to generate the equivalence point pH formula below:

- Ca = concentration of acid and Cb = concentration of base
- Kw = dissociation constant for water and Ka = for the acid
Note that when an acid neutralizes a base, the pH may or may not be neutral (pH = 7). The pH depends on the strengths of the acid and base.
4. After the equivalence point, the solution will contain two bases: the conjugate base of the acid and the strong base of the titrant. However, the base of the titrant is stronger than the conjugate base of the acid. Therefore, the pH in this region is controlled by the strong base. As such the pH can be found using the following:

Single formula. More accurately, a single formula that describes the titration of a weak acid with a strong base from start to finish is given below:


- φ = fraction of completion of the titration (φ < 1 is before the equivalence point, φ = 1 is the equivalence point, and φ > 1 is after the equivalence point)
- Ca, Cb = the concentrations of the acid and base respectively
- Va, Vb = the volumes of the acid and base respectively
- αA- = the fraction of the weak acid that is ionized
- Ka = the dissociation constant for the acid
- [H+], [OH-] = concentrations of the H+ and OH- ions respectively
This formula is somewhat cumbersome, but does describe the titration curve as a single equation.
See also
- TitrationTitrationTitration, also known as titrimetry, is a common laboratory method of quantitative chemical analysis that is used to determine the unknown concentration of an identified analyte. Because volume measurements play a key role in titration, it is also known as volumetric analysis. A reagent, called the...
- Titration curveTitration curveTitrations are often recorded on titration curves, whose compositions are generally identical: the independent variable is the volume of the titrant, while the dependent variable is the pH of the solution...
- Equivalence PointEquivalence pointThe equivalence point, or stoichiometric point, of a chemical reaction when a titrant is added and is stoichiometrically equal to the amount of moles of substance present in the sample: the smallest amount of titrant that is sufficient to fully neutralize or react with the analyte...
- Acid dissociation constantAcid dissociation constantAn acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
- Acid–base reaction
- Henderson-Hasselbalch

