
4-Nitroaniline
Encyclopedia
4-Nitroaniline, p-nitroaniline or 1-amino-4-nitrobenzene is an organic compound with the formula C6H6N2O2. It is an organic chemical compound
, consisting of a phenyl group attached to an amino group which is para
to a nitro group. The chemical structure of p-nitroaniline is shown at the right. This chemical is commonly used as an intermediate in the synthesis of dyes, antioxidants, pharmaceuticals and gasoline, in gum inhibitors, poultry medicines, and as a corrosion inhibitor.
. The key step in this reaction sequence is an electrophilic aromatic substitution
to install the nitro group para to the amino group. After this reaction, a separation must be performed to remove 2-nitroaniline, which is also formed in a small amount during the reaction.
, the first Azo dye:

Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
, consisting of a phenyl group attached to an amino group which is para
Arene substitution patterns
Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon.- Ortho, meta, and para substitution :...
to a nitro group. The chemical structure of p-nitroaniline is shown at the right. This chemical is commonly used as an intermediate in the synthesis of dyes, antioxidants, pharmaceuticals and gasoline, in gum inhibitors, poultry medicines, and as a corrosion inhibitor.
Synthesis
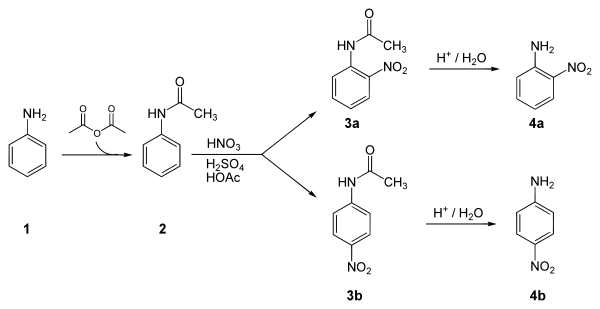
Below is an example synthesis of p-nitroaniline from anilineAniline
Aniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Being a precursor to many industrial chemicals, its main use is in the manufacture of precursors to polyurethane...
. The key step in this reaction sequence is an electrophilic aromatic substitution
Electrophilic aromatic substitution
Electrophilic aromatic substitution EAS is an organic reaction in which an atom, usually hydrogen, appended to an aromatic system is replaced by an electrophile...
to install the nitro group para to the amino group. After this reaction, a separation must be performed to remove 2-nitroaniline, which is also formed in a small amount during the reaction.

Applications
4-Nitroaniline is a starting material for the synthesis of Para RedPara Red
Para Red is a chemical dye. Chemically, the dye is similar to Sudan I. The dye was discovered in 1880 by von Gallois and Ullrich, and was the first azo dye. It dyes cellulose fabrics a brilliant red, but is not very fast. The dye can be washed away easily from cellulose fabrics if not dyed...
, the first Azo dye:


