2,6-Xylenol
Encyclopedia
2,6-Xylenol is a chemical compound which is one of the six isomer
s of xylenol
. It is also commonly known as 2,6-dimethylphenol (DMP).
2,6-Xylenol is a monomer
for poly(p-phenylene oxide)
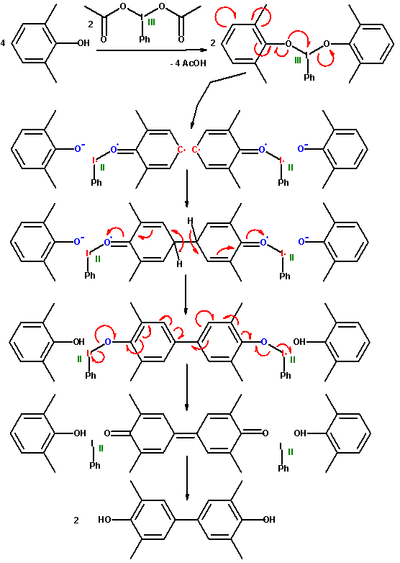
engineering resins through carbon - oxygen oxidative coupling. Carbon to carbon dimerization is also possible. In one study 2,6-xylenol is oxidized with iodosobenzene diacetate with an 5 fold excess of the phenol..
In the first step of the proposed reaction mechanism
the acetyl
groups in the iodine compound are replaced with the phenol. This complex dissociates into an aryl radical anion
and a phenoxy residue. The two aryl radicals recombine forming a new carbon carbon covalent bond
and subsequently lose two protons in a rearomatization step. The immediate reaction product is a diphenoquinone
as result of a one-step 4-electron oxidation. It is nevertheless possible to synthesize the biphenol
compound via a comproportionation of the quinone with xylenol already present. In this reaction sequence the hypervalent iodine reagent is eventually reduced to phenyliodine.
Isomer
In chemistry, isomers are compounds with the same molecular formula but different structural formulas. Isomers do not necessarily share similar properties, unless they also have the same functional groups. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical...
s of xylenol
Xylenol
Xylenol or dimethylphenol is an arene compound with two methyl groups and a hydroxyl group. 6 isomers exist of xylenol of which 2,6-xylenol with both methyl group in an ortho position with respect to the hydroxyl group is the most important...
. It is also commonly known as 2,6-dimethylphenol (DMP).
2,6-Xylenol is a monomer
Monomer
A monomer is an atom or a small molecule that may bind chemically to other monomers to form a polymer; the term "monomeric protein" may also be used to describe one of the proteins making up a multiprotein complex...
for poly(p-phenylene oxide)
Poly(p-phenylene oxide)
Poly or poly is a high-temperature thermoplastic. It is rarely used in its pure form due to difficulties in processing. It is mainly used as blend with polystyrene, high impact styrene-butadiene copolymer or polyamide.-History:Polyphenylene ether was discovered in 1956 by A. S...
engineering resins through carbon - oxygen oxidative coupling. Carbon to carbon dimerization is also possible. In one study 2,6-xylenol is oxidized with iodosobenzene diacetate with an 5 fold excess of the phenol..
In the first step of the proposed reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
the acetyl
Acetyl
In organic chemistry, acetyl is a functional group, the acyl with chemical formula COCH3. It is sometimes represented by the symbol Ac . The acetyl group contains a methyl group single-bonded to a carbonyl...
groups in the iodine compound are replaced with the phenol. This complex dissociates into an aryl radical anion
Radical ion
A radical ion is a free radical species that carries a charge. Radical ions are encountered in organic chemistry as reactive intermediates and in mass spectrometry as gas phase ions...
and a phenoxy residue. The two aryl radicals recombine forming a new carbon carbon covalent bond
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
and subsequently lose two protons in a rearomatization step. The immediate reaction product is a diphenoquinone
Quinone
A quinone is a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C– groups with any necessary rearrangement of double bonds," resulting in "a fully conjugated cyclic dione structure."...
as result of a one-step 4-electron oxidation. It is nevertheless possible to synthesize the biphenol
Biphenyl
Biphenyl is an organic compound that forms colorless crystals. It has a distinctively pleasant smell. Biphenyl is an aromatic hydrocarbon with a molecular formula 2...
compound via a comproportionation of the quinone with xylenol already present. In this reaction sequence the hypervalent iodine reagent is eventually reduced to phenyliodine.